HTML
-
Tropospheric aqueous-phase chemistry plays a key role in the formation of oxidizing species in the atmosphere (Herrmann et al., 2015). Iron is the most abundant transition metal element in tropospheric particles. It enters the atmosphere through various ways, such as sea spray (Guasco et al., 2014), combustion and industrial activities, and from the earth via wind (Liati et al., 2013; Li et al., 2016). Fenton reaction caused by the presence of iron is widespread in the atmosphere (Deguillaume et al., 2004). Some studies (Nguyen et al., 2013; Chu et al., 2014) have indicated that the Fenton reaction in the atmosphere mainly happens in the aqueous phase. Some estimates suggest that Fenton reaction involving iron might account for ~30% of the ·OH production in fog droplets (Deguillaume et al., 2005). The oxidizing ability contributed from Fenton reaction is highly dependent on pH, iron concentration, and the kind and concentration of organic compounds that form complexes with iron (Zuo and Hoigne, 1992; Weller et al., 2013b, a). Notably, the formation of iron complexes have a significant effect on the photochemical reaction pathway. Iron can complex with oxalic acid, which was the most abundant dicarboxylic acid in aqueous aerosol (Sorooshian et al., 2006; Legrand et al., 2007), and the ferric oxalate complex (Fe(III)-Ox) can efficiently produced oxidized species by photo-reduce Fe(II) under relevant atmospheric condition (Thomas et al., 2016). Oxalic acid is an ultimate end product in the oxidation of organic compounds in atmosphere such as isoprene and aromatic hydrocarbons (Boreddy et al., 2017). Oxalic acid could also come from incomplete combustion. One simulation has shown that at least 50% of Fe(III) is bound by oxalic acid during both cloud and deliquescent particle periods because of their strong complexing coefficients (Weller et al., 2014), and 99% of oxalic acid is consumed by photolysis. Field observations have also shown a significant negative correlation between atmospheric oxalic and Fe(III) concentrations (Sorooshian et al., 2013). Photolysis of Fe(III)-Ox in the atmosphere is extensive. Thus, it is necessary to evaluate its effect on atmospheric oxidation. Although the photochemistry of Fe(III)-Ox has been studied extensively, knowledge of its impact on the aging of atmospheric organic matter, especially a volatile organic compound (VOC) with a low boiling point in the aqueous phase, is still inadequate (Thomas et al., 2016; Pang et al., 2019). There is a potential for secondary aerosol formation when a VOC with a low boiling point transfers to a product with a higher boiling point.
Isoprene (2-methyl-1,3-butadiene, C5H8) is a widespread biogenic hydrocarbon (Seinfeld and Pandis, 2016) that relates closely to photosynthetic activity in plants. Its main oxidation product is methacrolein (C4H6O, MACR), which occupies about 22% of the yield of isoprene. MACR is a model compound in photochemical experiments. Although MACR has a small Henry coefficient (
$ {\mathrm{H}}_{\mathrm{m}\mathrm{e}\mathrm{t}\mathrm{h}\mathrm{a}\mathrm{c}\mathrm{r}\mathrm{o}\mathrm{l}\mathrm{e}\mathrm{i}\mathrm{n}}=6.5\;\mathrm{M}\;{\mathrm{a}\mathrm{t}\mathrm{m}}^{-1} $ ), its concentration in the aqueous phase is found to be much higher than expected based only on its Henry coefficient (van Pinxteren et al., 2005). Thus, MACR is a typical representative VOC in the aqueous phase. The oxidation mechanism of MACR has been investigated previously in many studies (Zhang et al., 2010; Liu et al., 2012; Schöne et al., 2014; Giorio et al., 2017), allowing for easy comparisons.This study explores the aqueous photo-Fenton reaction involved in the oxidation of MACR in the presence of Fe(III)-Ox. The effects of oxalate concentration, Fe(III) concentration, MACR concentration, and pH are analyzed. The main long-lived oxidation species, Fe(III) and H2O2, in photo-Fenton reaction are measured. The kinds and abilities of free radicals involved in the reaction with MACR are evaluated. The photodegradation mechanism and the possible oxidative products are studied by liquid-chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS). The oxidation products (namely, small organic acids) are analyzed by ion chromatograph (IC). Furthermore, the absorbance of the aqueous solution is tracked by a UV-vis spectrometer. The results obtained in this study will provide a reference for predicting the influence of low boiling-point VOCs in photooxidation with Fe(III)-carboxylic complexes in atmospheric water.
-
MACR (95%) and potassium oxalate (99.98%) were purchased from Shanghai Aladdin Bio-Chem Technology Co. Ltd. Ferric perchlorate, perchloric acid (70%), ferrozine (97%), 4-hydroxyphenylacetic acid (99%), and peroxidase (from horseradish, RZ>2.5) were purchased from Sigma-Aldrich. Analytical grade
$ {\mathrm{C}\mathrm{H}}_{3}\mathrm{C}\mathrm{O}\mathrm{O}\mathrm{H} $ ,$ {\mathrm{C}\mathrm{H}}_{3}\mathrm{C}\mathrm{O}\mathrm{O}\mathrm{N}\mathrm{a} $ , NaOH, hydroxylamine hydrochloride, phenazine, and ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) were purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized (DI) water was utilized in all experiments. -
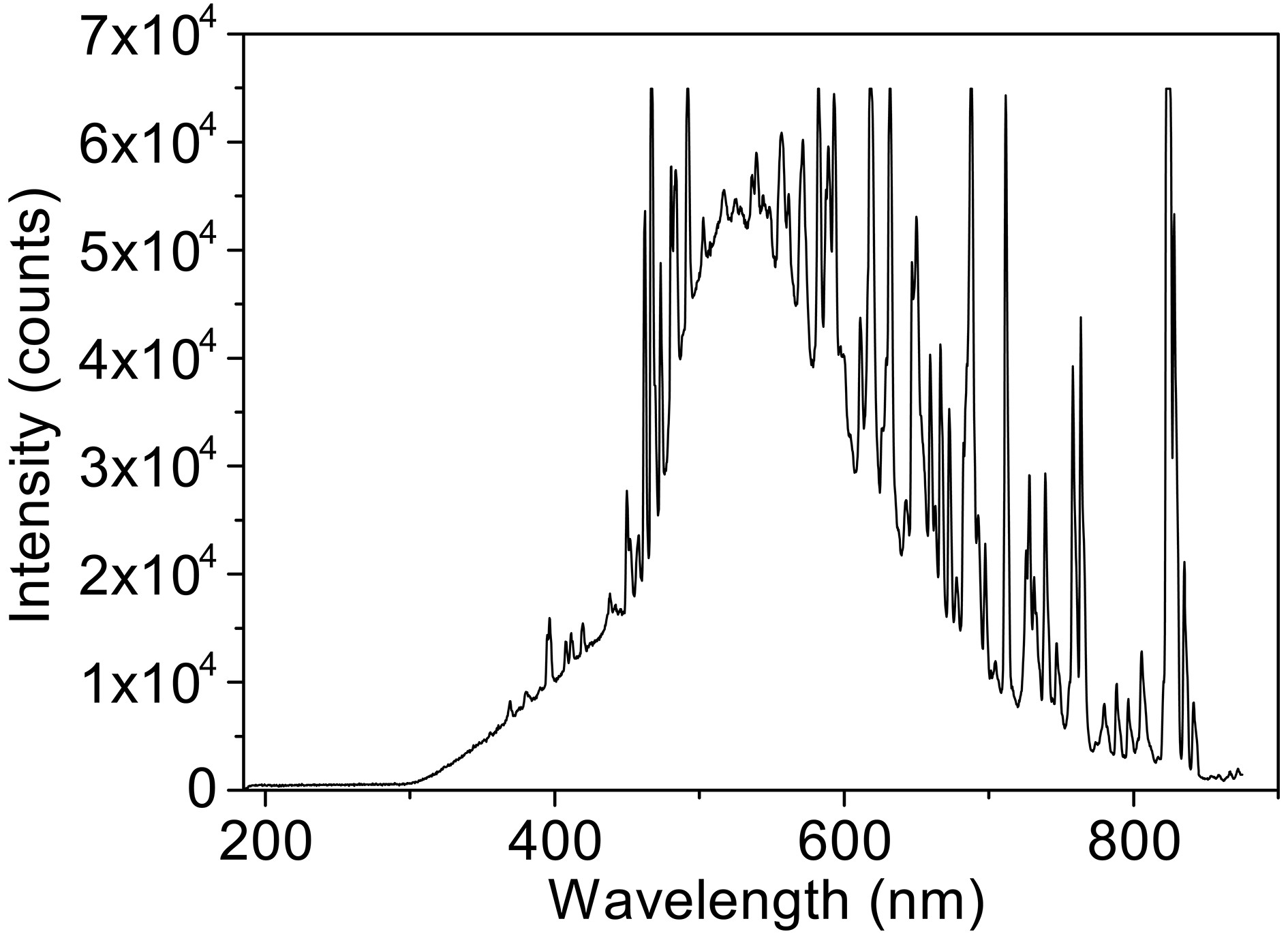
Photolysis reaction was performed in a photochemical reactor (XPA-VII, Xujiang electromechanical plant, Nanjing, China) equipped with a 500 W Xenon lamp combined with a 290 nm cut-off filter as simulated solar light. The Xenon lamp spectrum received by the reaction solution is shown in Fig. A1 in the Appendix. The temperature of the reactor was maintained at 25°C ± 2°C by cooling water. The pH was adjusted by
$ \mathrm{H}{\mathrm{C}\mathrm{l}\mathrm{O}}_{4} $ . A 20 mL solution was prepared under constant magnetic stirring and reacted in a 50 mL cylindrical quartz tube sealed with a rubber stopper. Before sealing, the aqueous solution, which did not contain MACR, was stirred in the dark for 10 min to achieve oxygen saturation. Then, 1 mL of the solution was taken out through the rubber stopper using a syringe and filtered through a poly tetra fluoroethylene (PTFE) 0.22 μm filter. -
GC-FID (Agilent 7890A, USA) was used to analyze the MACR concentration. It was equipped with a semi-polar capillary column (CNW CD-5MS 30 m × 0.25 mm × 0.25 μm, Germany), which allowed the injection of aqueous phase samples. The GC injector and detector were heated at 250°C. Nitrogen gas was used as carrier gas at 1 mL min−1 with a 1/10 split. The oven temperature program was 40°C for 4 min, raised by 10°C min−1 up to 120°C, and 120°C for 5 min (El Haddad et al., 2009; Liu et al., 2009).
An IC (DIONEX ICS-3000, Thermo Fisher, USA) assembled with AG11-HC guard column and AS11-HC analytical column was used to measure organic acid anions (Zhou et al., 2018). The pH of the aqueous solution containing iron ion was adjusted to 10. Then, the solution was passed through a 0.22 μm PTFE filter and an H-type pretreatment filter to remove the iron.
The concentrations of Fe(II) and total dissolved iron were determined by using a spectrophotometry methodology with ortho-phenanthroline and hydroxylamine (Sastry and Rao, 1996). Hydrogen peroxide concentrations were determined by the fluorescence method developed by Lazrus et al. (1985) after sample mixing with EDTA-2Na. Solution pH was measured using a pH meter (METTLER TOLEDO FE20, Switzerland). UV-vis spectra of the solutions were tracked by UV-vis spectrometer (Scinco Nano MD, SCINCO, South Korea).
Reaction byproducts were identified with a LC-QTOF-MS system (Agilent 6540, USA) equipped with an electrospray ionization source, and analysis was performed under negative ionization mode (Lian et al., 2017).
2.1. Materials
2.2. Photolysis experiments
2.3. Analytical procedures
-
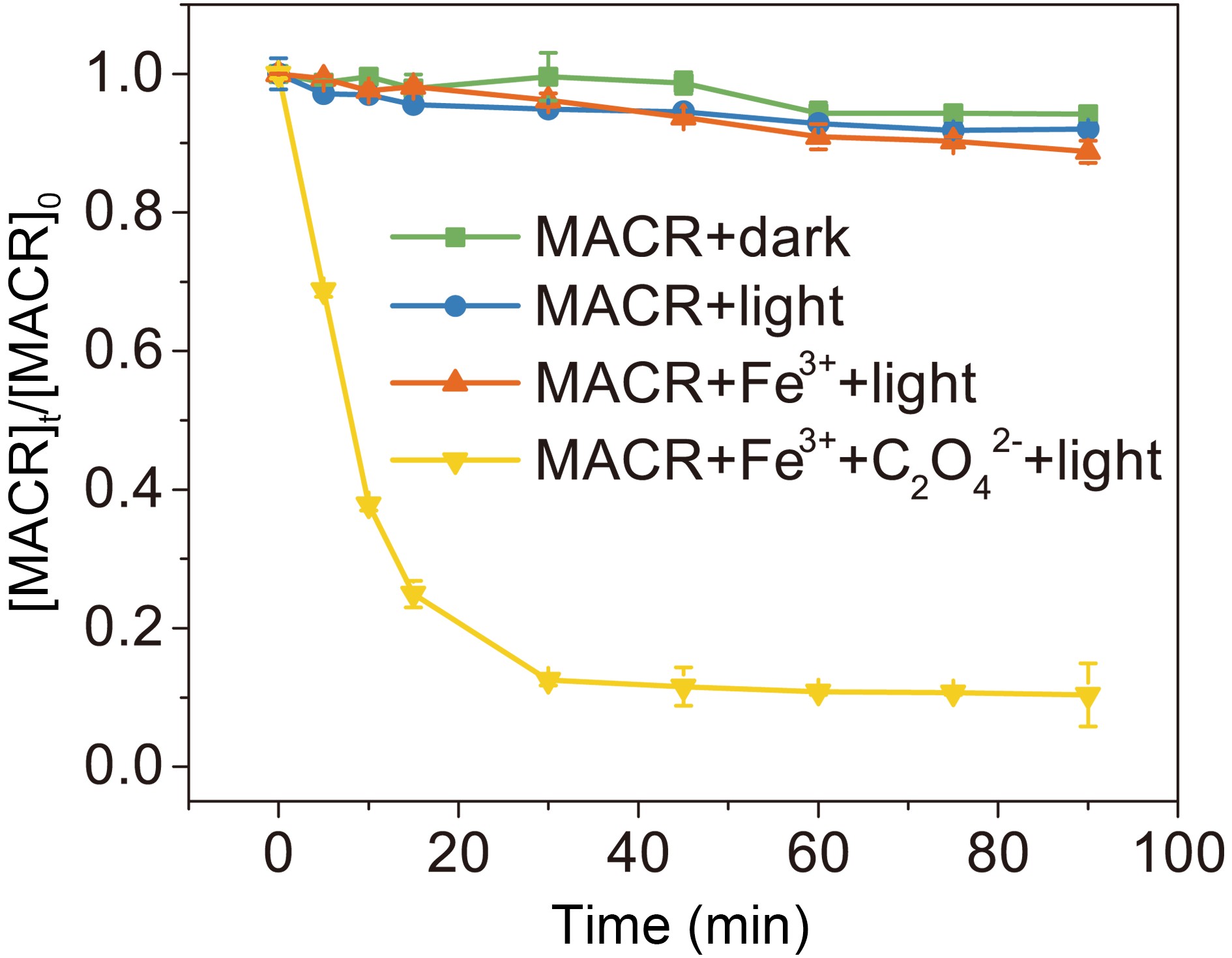
As seen in Fig. 1, MACR shows little change under dark conditions. The small Henry coefficient of MACR keeps it mostly distributed in the aqueous solution, and errors caused by gas-liquid distribution are ignored in subsequent experiments. No obvious direct photolysis of MACR is found under the simulated sunlight conditions (λ > 290 nm) because MACR’s absorbance peak at 375 nm is low (Fig. 2). In the presence of Fe(III), MACR is oxidized about 7% in 90 min.
$ {\mathrm{F}\mathrm{e}\left(\mathrm{O}\mathrm{H}\right)}^{2+} $ is the dominant Fe(III) hydroxide complex in the aqueous solution with pH of 4, and it is expected to undergo photolysis to generate ·OH according to Eqs. (1−3) and then react with MACR.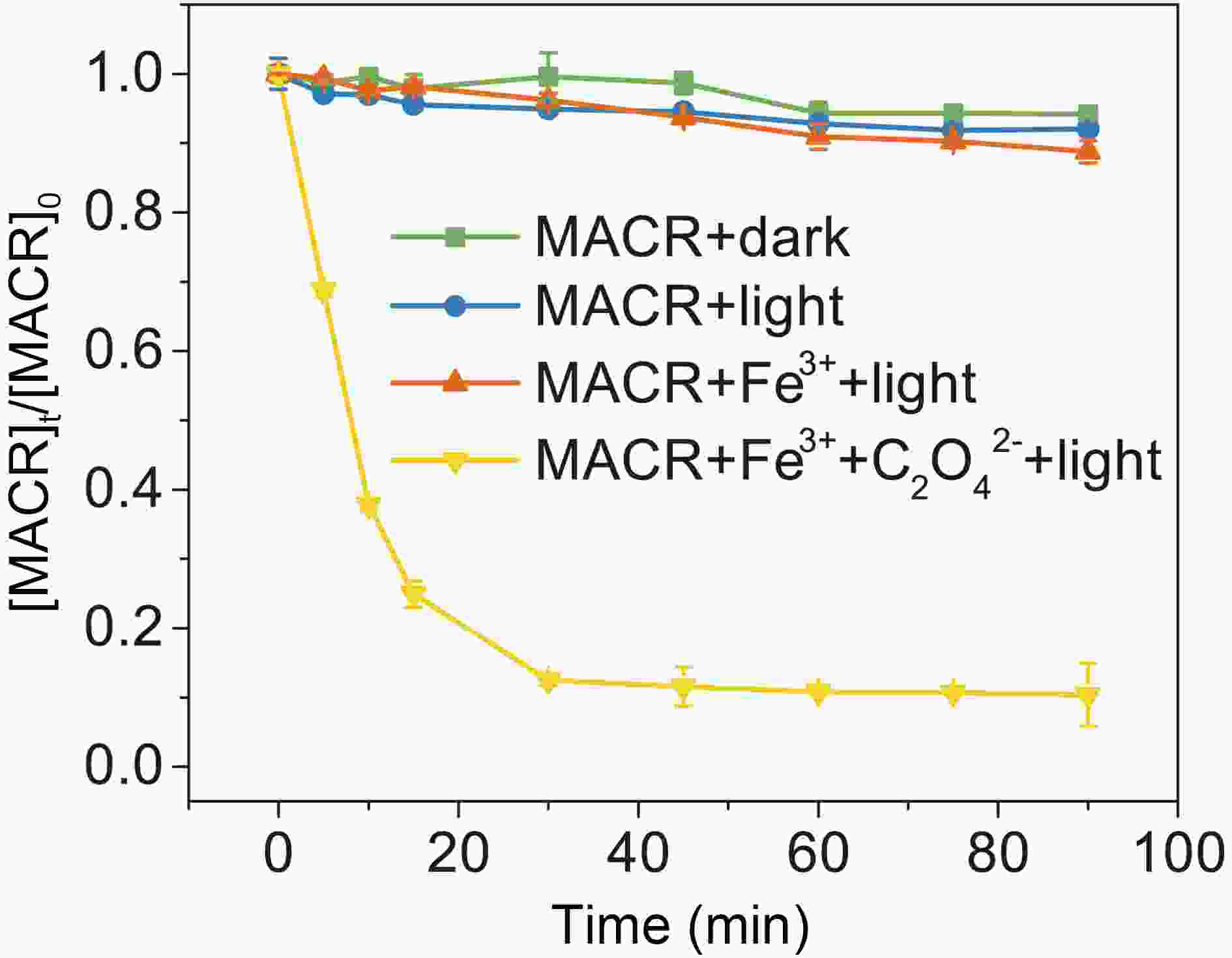
Figure 1. Control experiments of MACR oxidation ([MACR]0 = 500 μM, [Fe(III)]0 = 100 µM, [Oxalate]0 = 1000 µM, pH = 4.0 ± 0.1, air-saturated solution).
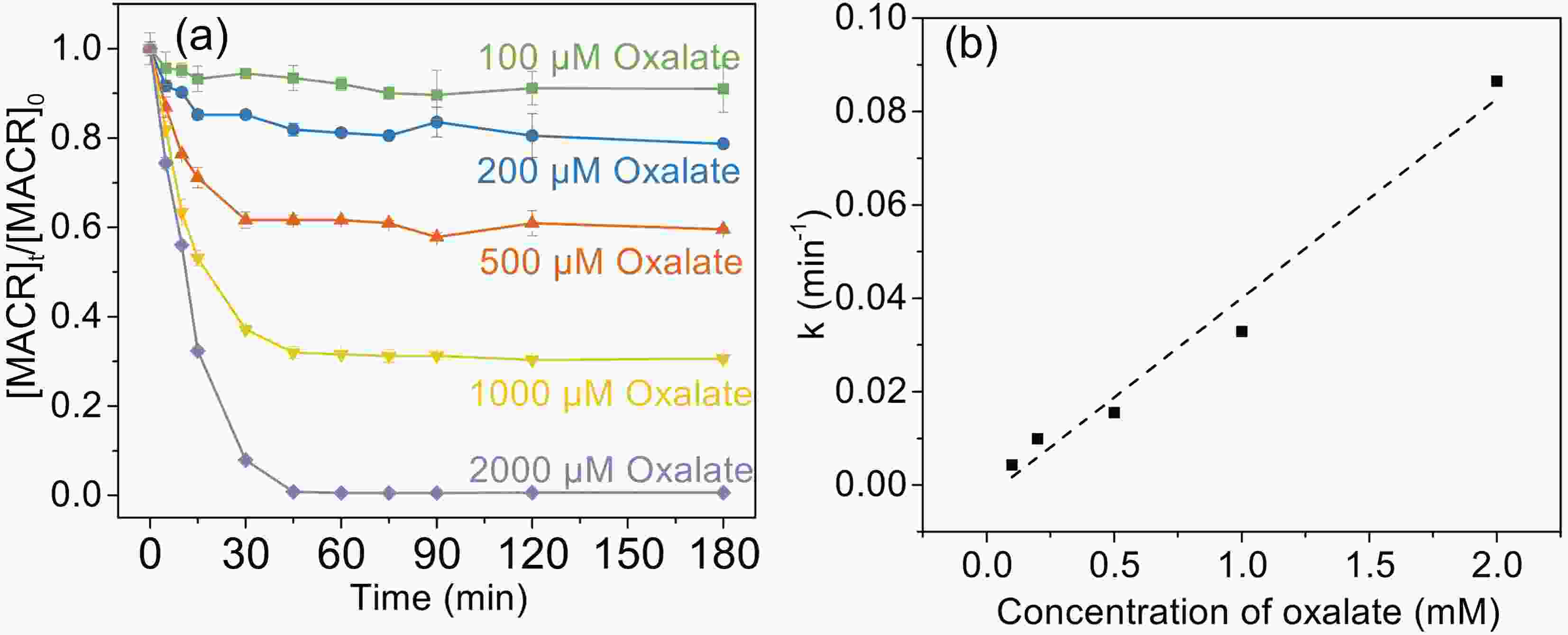
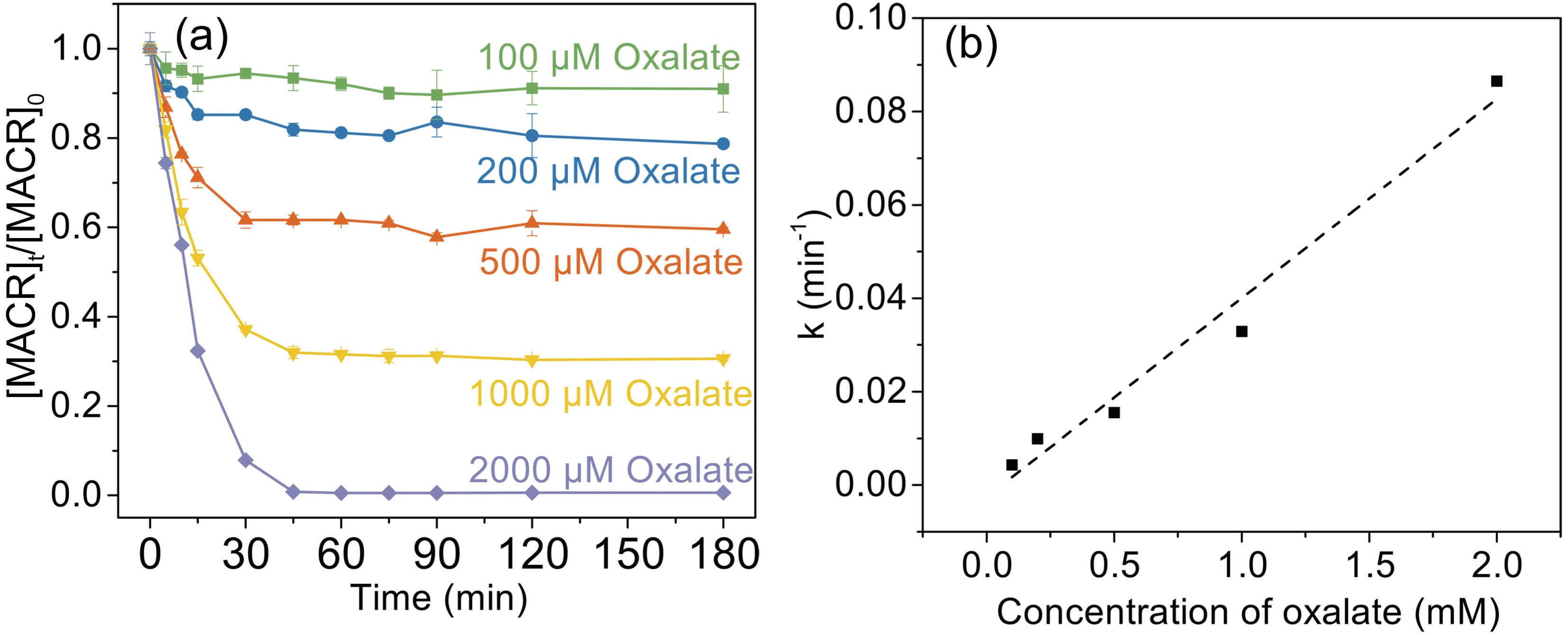
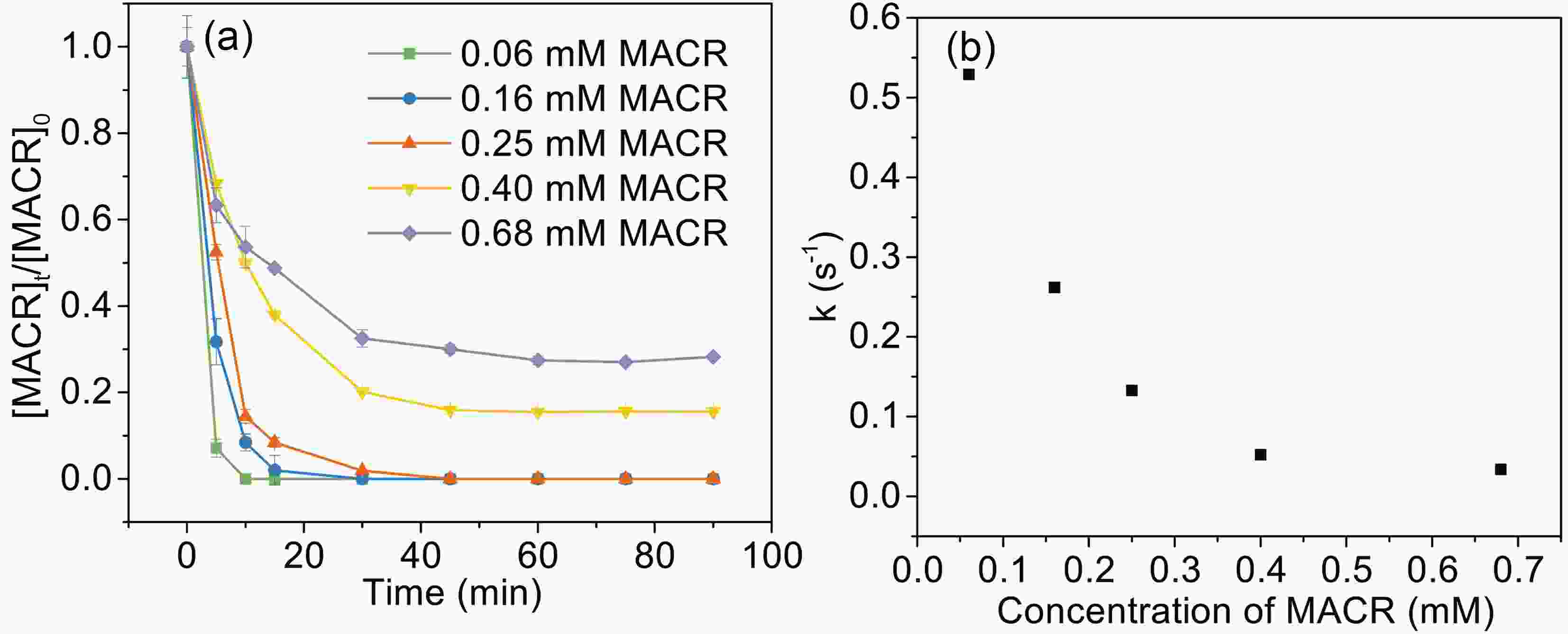
Figure 2. (a) Effect of oxalate concentration on the photooxidation of MACR in the Fe(III)-Ox system; (b) The reaction rate constant calculated by the pseudo-first-order kinetics variation with the concentration of oxalate ([Oxalate]0 = 1000 µM, [MACR]0 = 500 µM, pH 4.0 ± 0.1, air saturated solution).
where h is Planck constant, v is frequency of light.
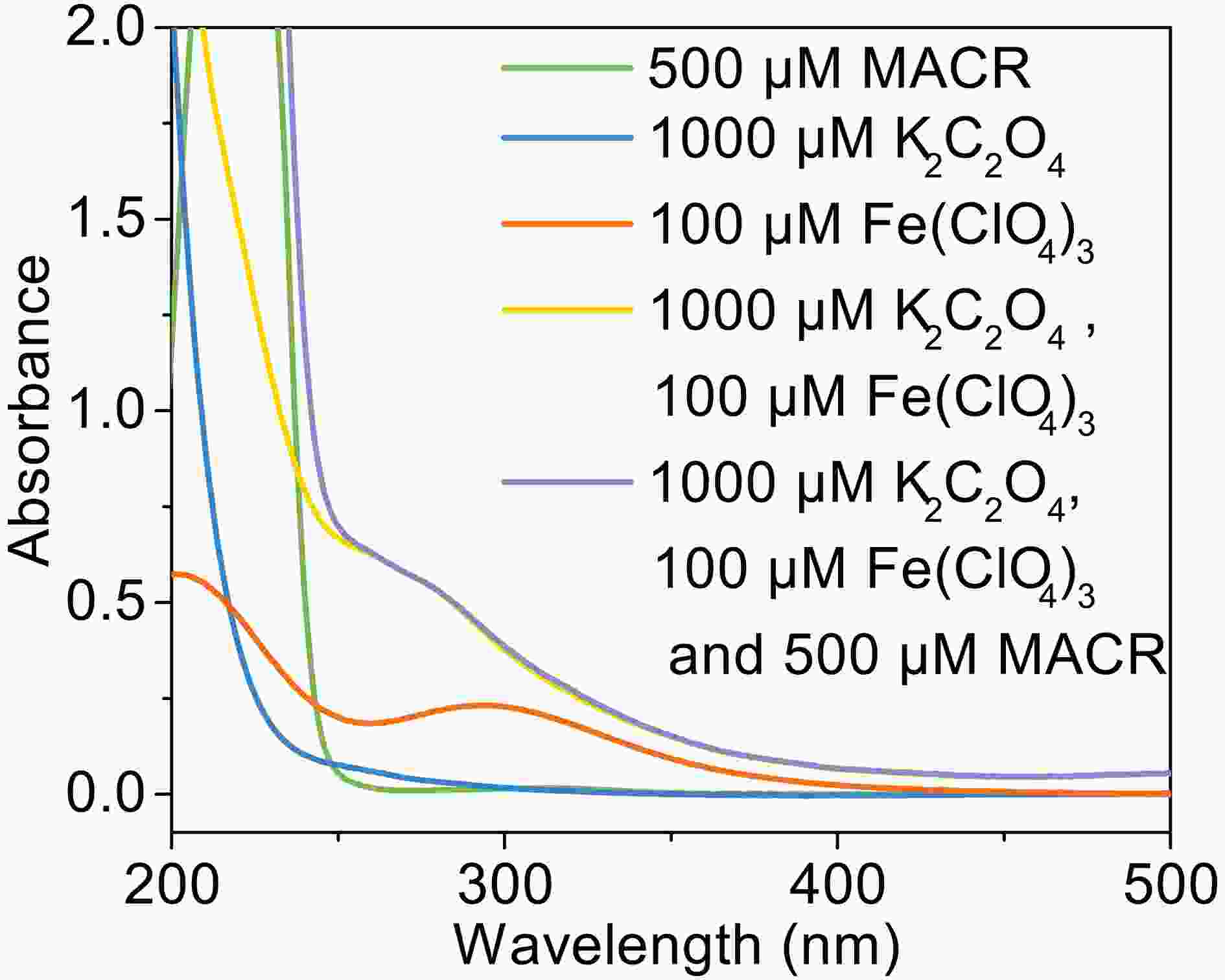
In the presence of Fe(III)-Ox (Fig. 1), the oxidation rate of MACR rapidly increases, and 80% MACR is oxidized in 60 min. This result occurs because Fe(III)-Ox forms and the efficiency of Fenton-like reaction is accelerated. As shown in Fig. A2, Fe(III)-Ox has more obvious absorption peaks in the sunlight range compared to the Fe(III) solution. Quantum yields (at 293 K, pH = 4.0) for the photolysis of
$ {\mathrm{F}\mathrm{e}\left(\mathrm{O}\mathrm{H}\right)}^{2+} $ at 313 nm and 360 nm are 0.14 ± 0.04 and 0.017 ± 0.003 (Faust and Hoigné, 1990), respectively.$ {\mathrm{F}\mathrm{e}\left(\mathrm{O}\mathrm{H}\right)}^{2+} $ has weak absorption in the solar UVA-visible region (Knight and Sylva, 1975). Quantum yields for$ {\left[\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{3}\right]}^{3-} $ at 313 nm and 366 nm are 2663 ± 37 and 709 ± 10, respectively, and quantum yields for$ {\left[\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}\right]}^{-} $ at 313 nm and 366 nm are 2055 ± 111 and 1.17 ± 1.46 (Weller et al., 2013b), respectively, which are much higher than$ {\mathrm{F}\mathrm{e}\left(\mathrm{O}\mathrm{H}\right)}^{2+} $ , indicating that higher photoactivity produces more radicals. -
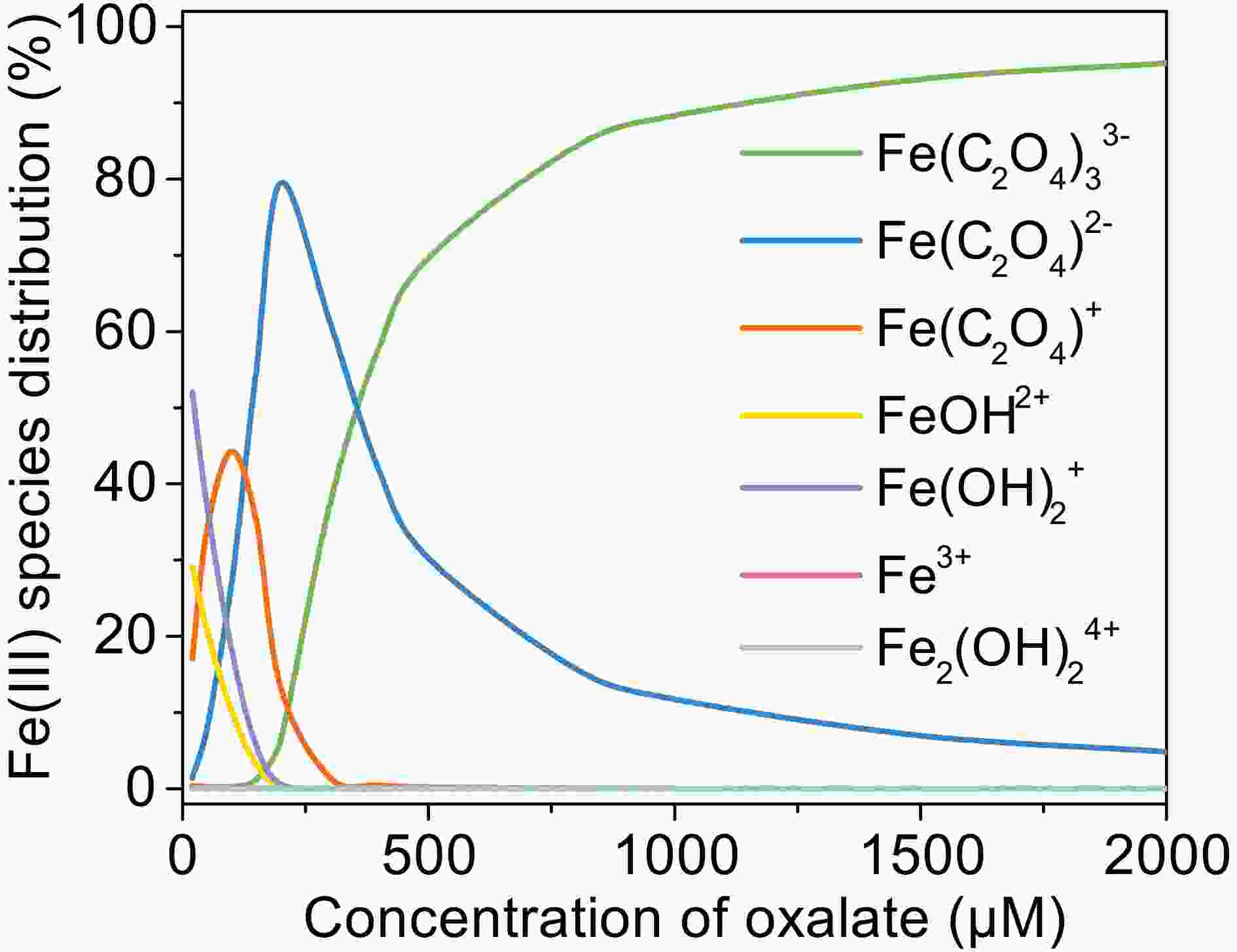
The effect of oxalate concentration is investigated in the range from 100 μM to 2000 μM. As shown in Fig. 2a, the oxidation rate of MACR increases with increasing oxalate concentration. The pseudo-first-order oxidation rate of MACR has an increase of nearly equal proportion with increasing oxalate concentration from 100 μM to 2000 μM (Fig. 2b). Approximately 80% MACR is oxidized when oxalate concentration is 1000 μM. Oxalate at 2000 μM leads to the oxidation of all MACR, though this concentration is excessive. However, the oxidation rate corresponding to 2000 μM oxalate still increases linearly, and the inhibitory effect caused by excess oxalate is not observed in Fig. 2b. This result may be due to the low second-order reaction rate constant of oxalate and ·OH.
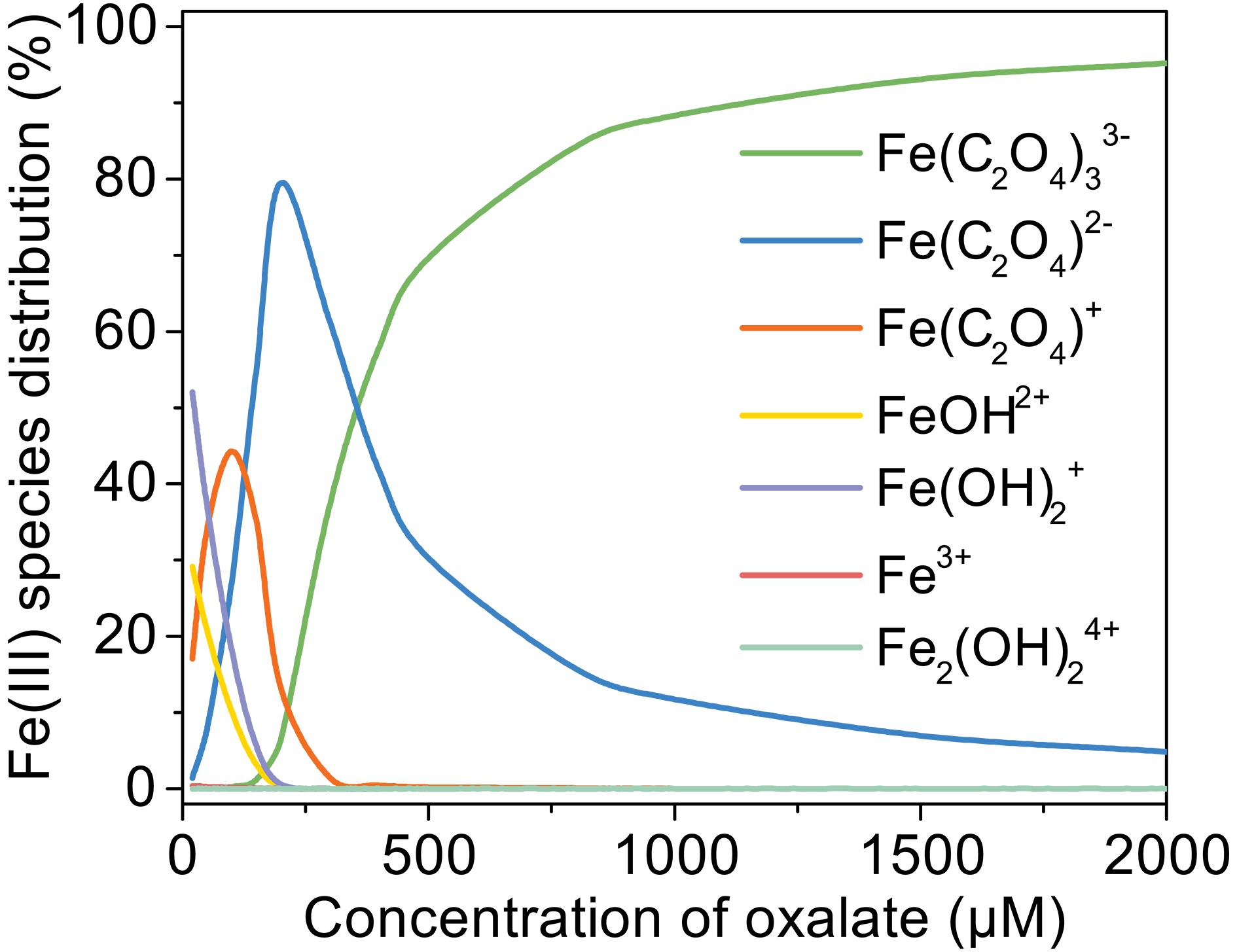
The increasing degradation efficiency of MACR is due to the formation of Fe(III)-Ox, which is photolyzed to yield oxalate radicals (
${\mathrm{C}}_{2}\rm{O}_{\small 4}^{\overline {\,\cdot\,}}$ ) and ·OH in the presence of dissolved oxygen (Eqs. 4−10).Different ratios of oxalate and ferric form different complexes, and quantum yields for
$ {\left[\mathrm{F}\mathrm{e}{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}\right]}^{-} $ are larger than$ {\left[\mathrm{F}\mathrm{e}{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{3}\right]}^{3-} $ under simulated sunlight (Weller et al., 2013a), which indicates that$ {\left[\mathrm{F}\mathrm{e}{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}\right]}^{-} $ has stronger photoactivity and can produce more active radicals. According to Fig. A3, the proportion of$ {\left[\mathrm{F}\mathrm{e}{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}\right]}^{-} $ increases first then decreases with increasing oxalate concentration from 100 μM to 2000 μM, while$ {\left[\mathrm{F}\mathrm{e}{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{3}\right]}^{3-} $ increases monotonously and becomes the main complex when the concentration of oxalate is above 500 μM. The different proportions of the complex also cause the difference in the photoactivity of the solution. However, the oxidation rate of MACR keeps increasing monotonically with increasing oxalate concentration, which may be due to the simultaneous existence of multiple complexes, resulting in insignificant differences in photoactivity. -
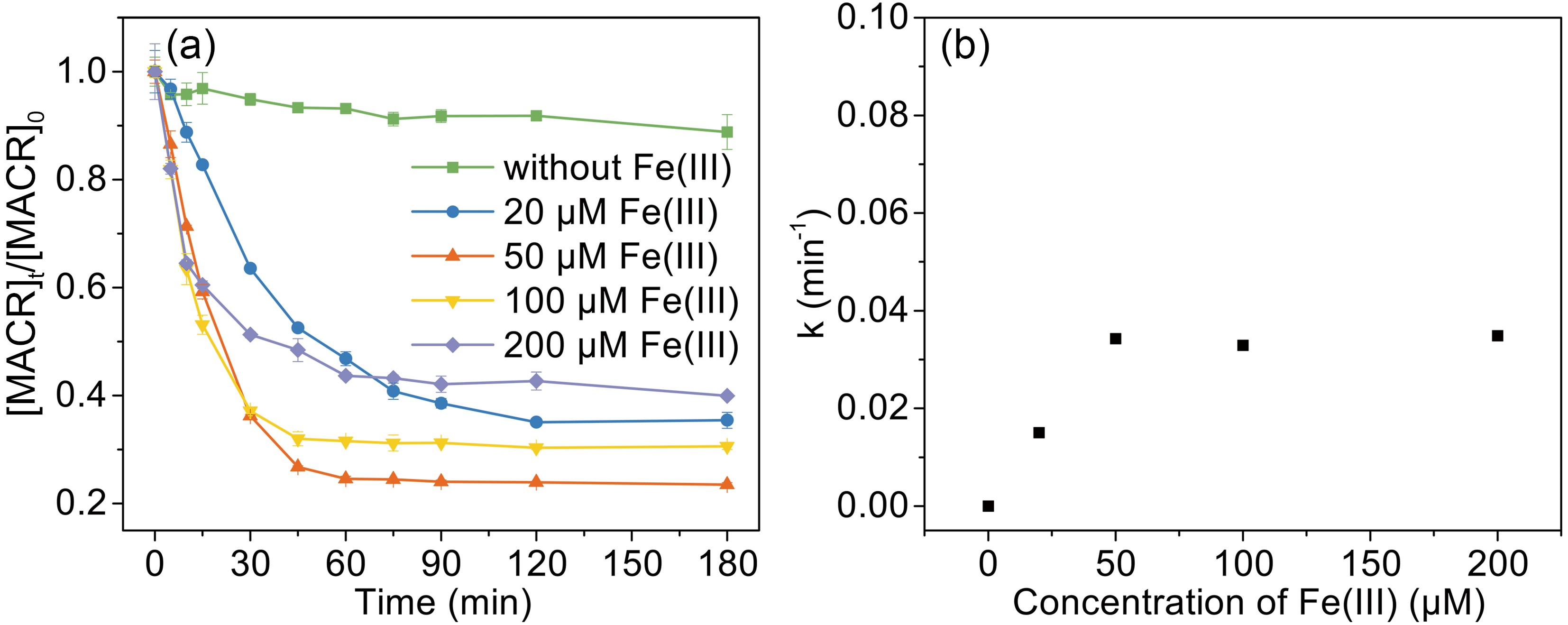
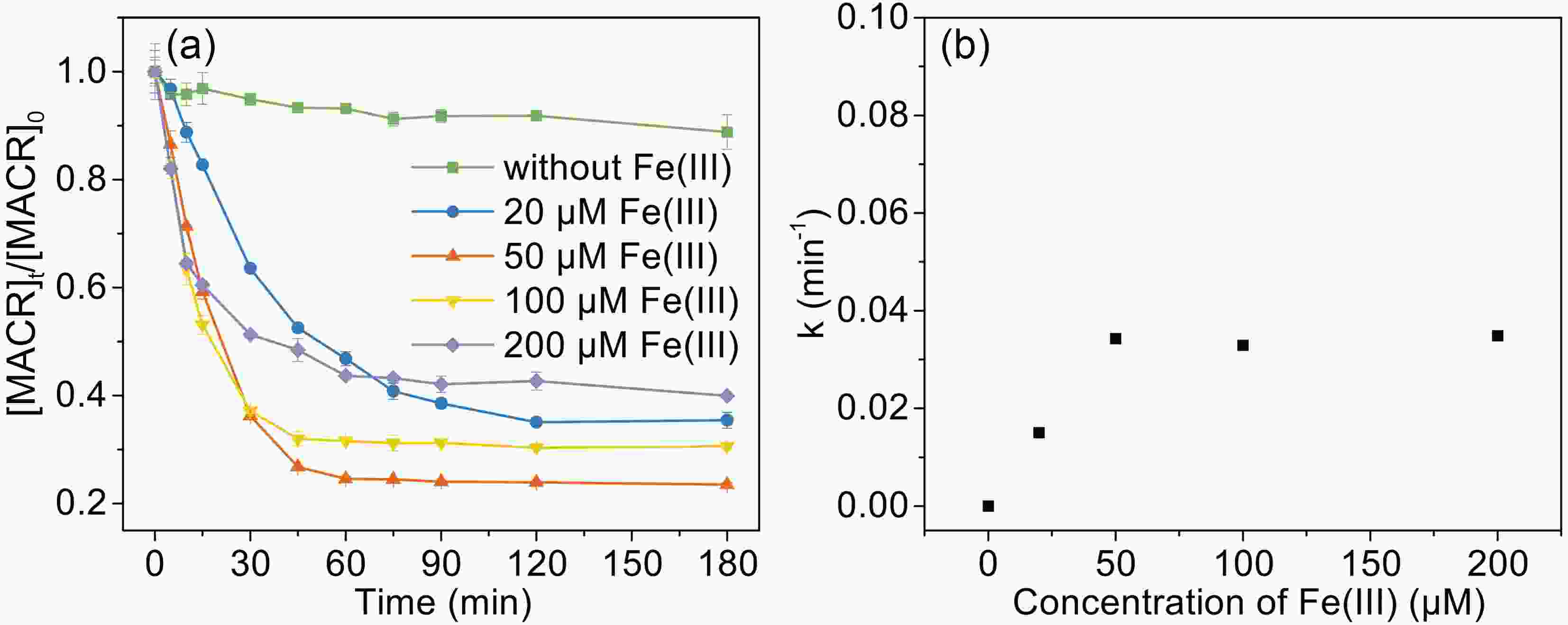
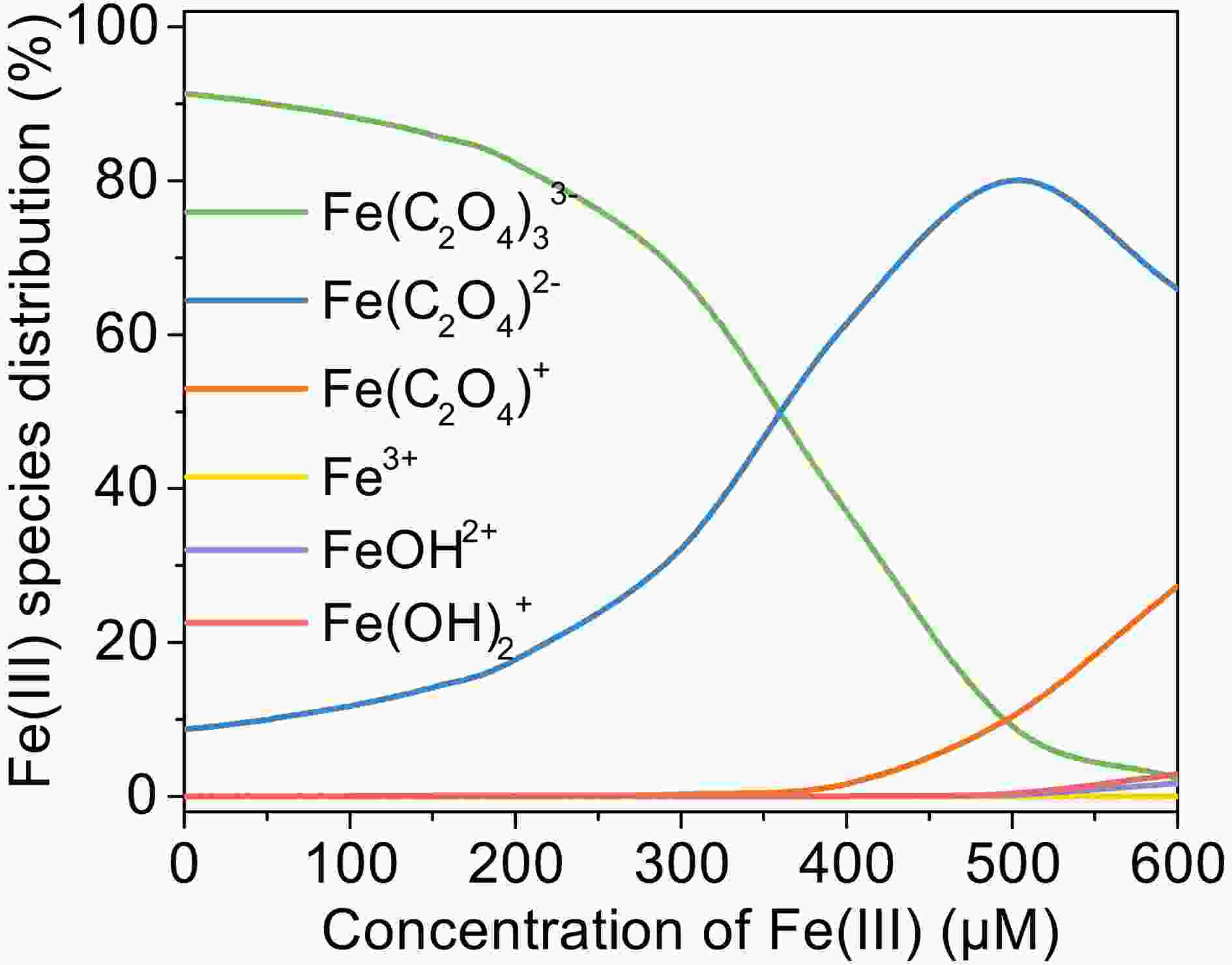
To investigate the effect of Fe(III) concentration on the photooxidation of MACR, the Fe(III) concentration was varied from 20 μM to 200 μM during the reaction. As shown in Fig. 3, the photooxidation rate of MACR increases with increasing Fe(III) concentration from 20 μM to 200 μM in the first 60 min. This is because the complex species become different when the concentration ratio between ferric and oxalate is changed. The
$ {\left[\mathrm{F}\mathrm{e}{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}\right]}^{-} $ with higher photoactivity is the main form when oxalate concentration is low, and more$ {\left[\mathrm{F}\mathrm{e}{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{3}\right]}^{3-} $ is produced with increasing oxalate concentration (Fig. A4). As the reaction time is extended, high Fe(III) concentration has a negative effect on MACR oxidation. The reason for this might be the consumption of${\mathrm{C}}\rm{O}_{\small 2}^{\overline {\,\cdot\,}}$ under relatively high Fe(III) concentration conditions. Normally,${{\mathrm{C}\rm{O}_{\small 2}^{\overline {\,\cdot\,}}} }$ reacts with dissolved$ {\mathrm{O}}_{2} $ and causes formation of$ {\mathrm{H}}_{2}{\mathrm{O}}_{2} $ and ·OH at low Fe(III)-Ox concentration. However, the majority of$\rm{CO}_{\small 2}^{\overline {\,\cdot\,}}$ interacts with Fe(III)-Ox, resulting in the formation of Fe(II) and$ {\mathrm{C}\mathrm{O}}_{2} $ (Eq. 11) with a high concentration of Fe(III)-Ox. The formation of OH radicals is inhibited in this situation. -
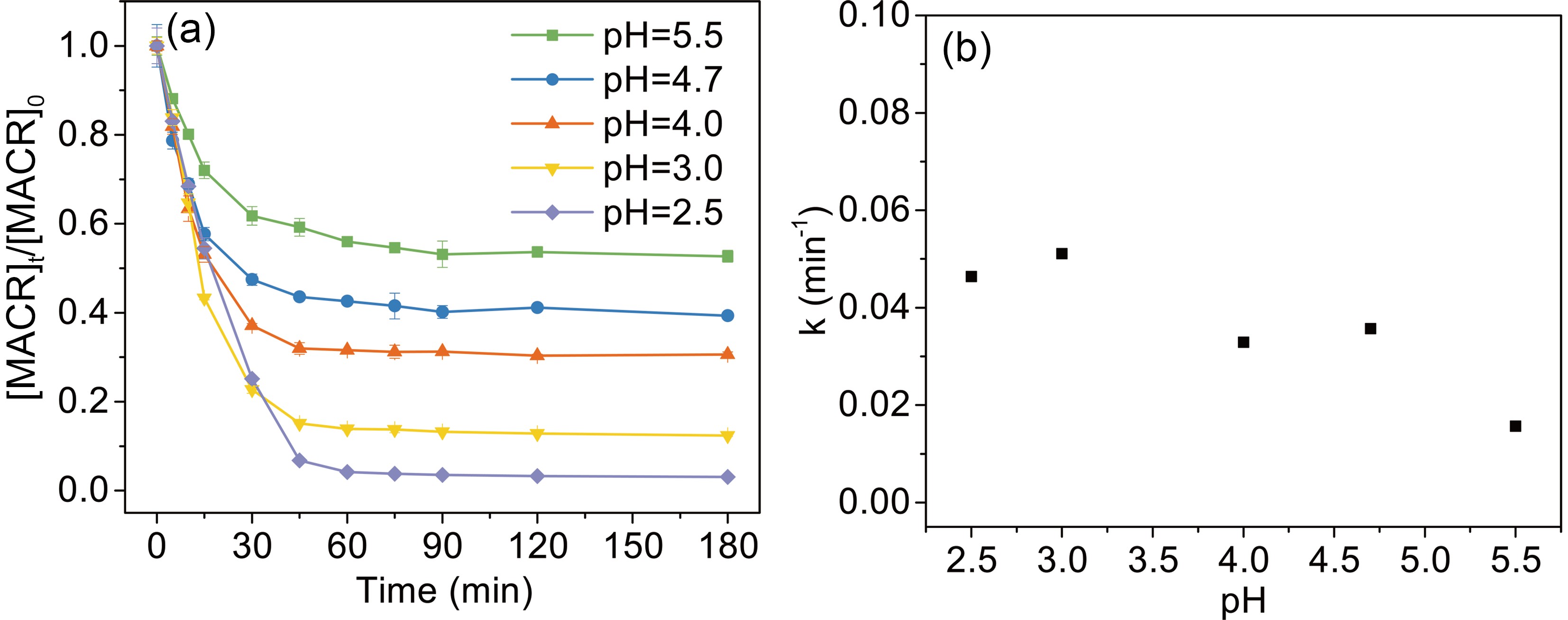
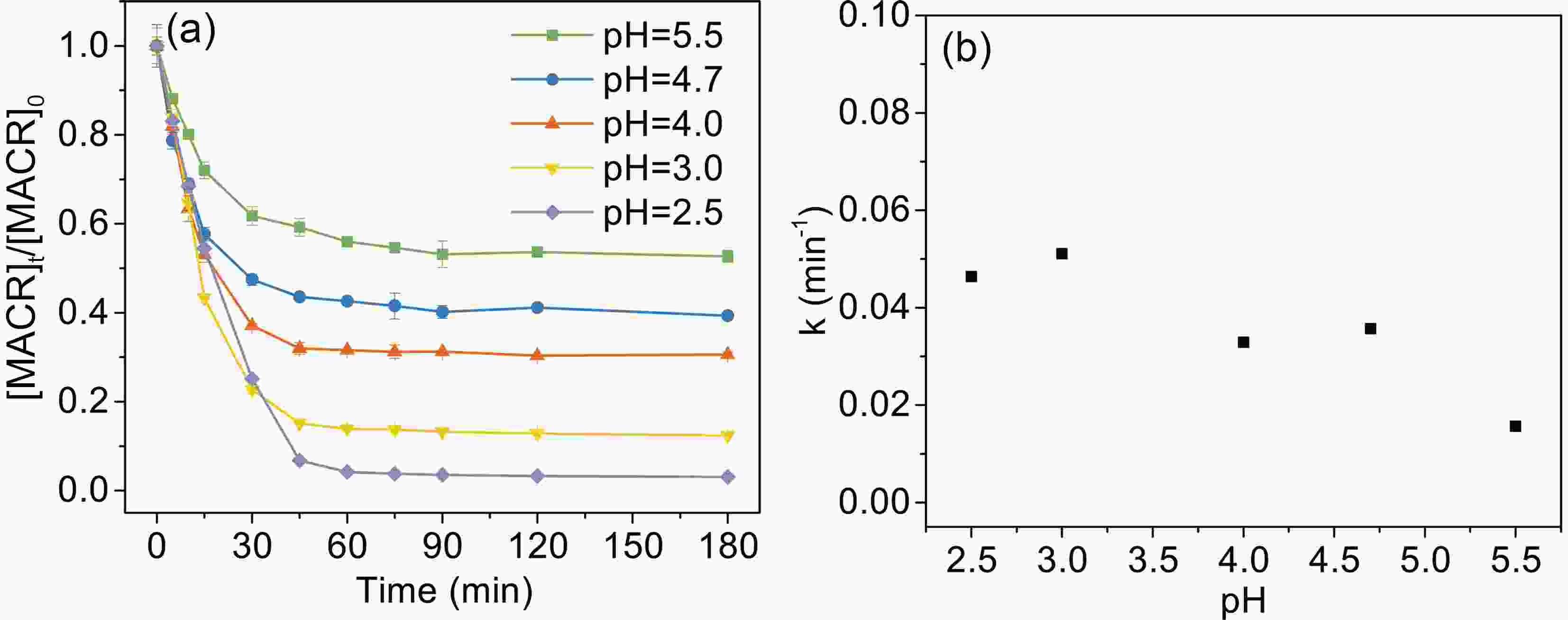
Solution pH has an important effect on the speciation and reactivity of Fe(III)-Ox. The typical pH value in the atmosphere is from 2.5 to 5.5. As shown in Fig. 4, the photooxidation rates of MACR decrease with increasing pH from 2.5 to 5.5.
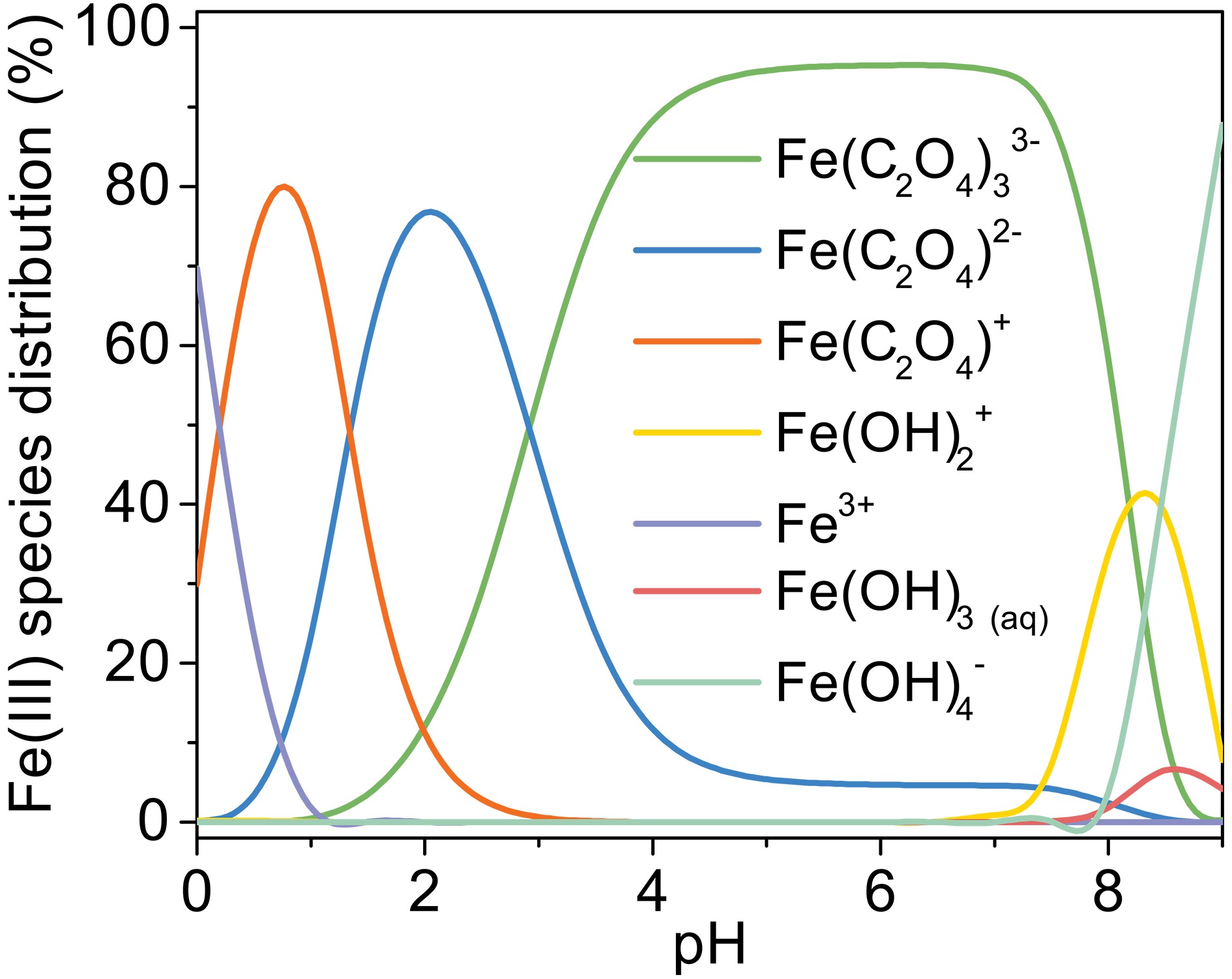
$ {\left[\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}\right]}^{-} $ is the major complex in pH 2.5 and then decreases with increasing pH value. Then,$ {\left[\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{3}\right]}^{3-} $ becomes the dominant complex. Considering that$ {\left[\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{3}\right]}^{3-} $ and$ {\left[\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}\right]}^{-} $ have stronger photoactivity than$ [{\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right)\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)]}^{+} $ and$ {\mathrm{F}\mathrm{e}}^{3+} $ (Weller et al., 2013b), pH affects the photo-reactivity by changing the complex forms. Moreover, pH also affects the reactions between$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ /$ {{\mathrm{H}\mathrm{O}}_{2}^{\,\cdot\,}} $ and Fe(II) (Eq. 9), and these reactions contribute most of the H2O2 in the Fe(III) oxalate system.$ {{\mathrm{H}\mathrm{O}}_{2}^{\,\cdot\,}} $ is the dominant species at low pH, and then it transfers to$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ with increasing pH (Eq. 8) (Zuo and Hoigne, 1992).$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ can react to the H2O2 form faster than$ {{\mathrm{H}\mathrm{O}}_{2}^{\,\cdot\,}} $ . So, these two factors allow low pH to have a positive effect on MACR oxidation. -
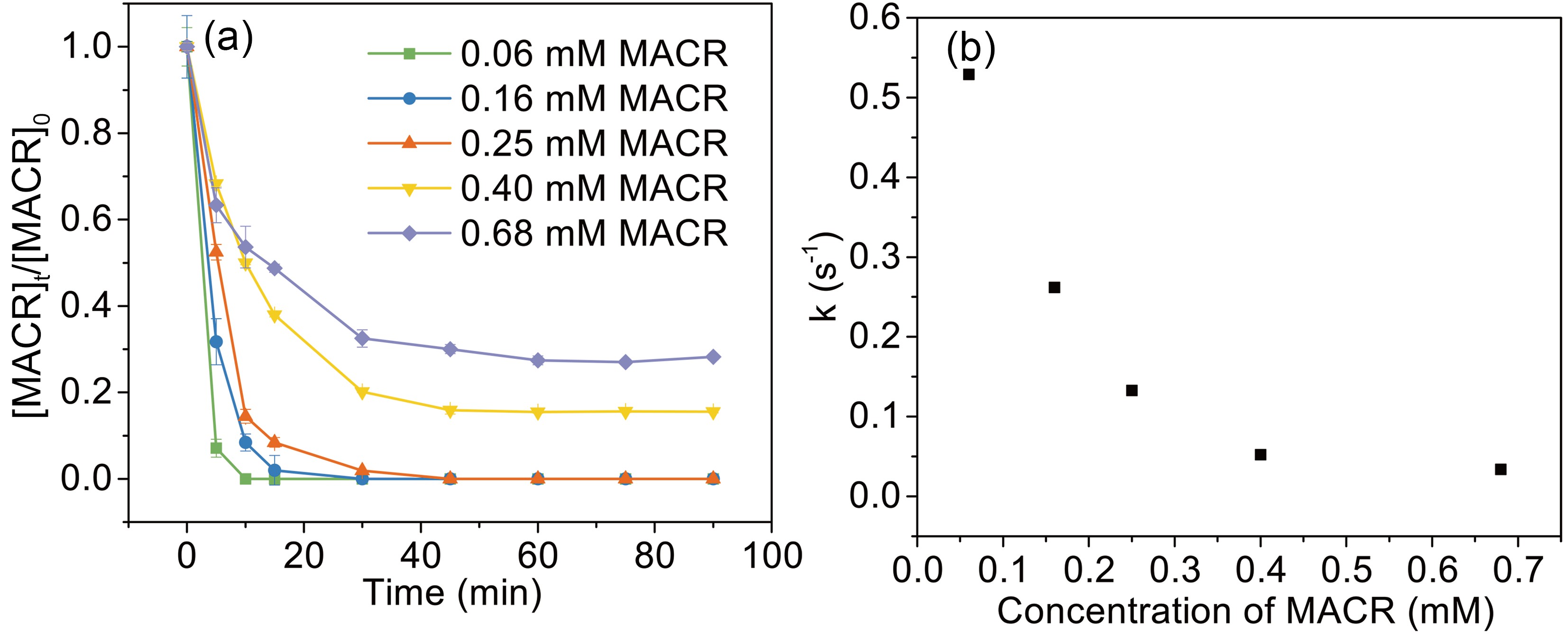
The influence of initial MACR concentration (from 60 μM to 680 μM) is investigated (Fig. 5). The oxidation rates of MACR increase with decreasing MACR concentration, and 60 μM, 160 μM, and 250 μM MACR are 100% oxidized during 45 min. However, a certain amount of MACR remains when MACR concentration is high (such as 400 μM and 680 μM). This is because oxalate is depleted and is unable to produce enough ·OH to completely oxidize MACR.
-
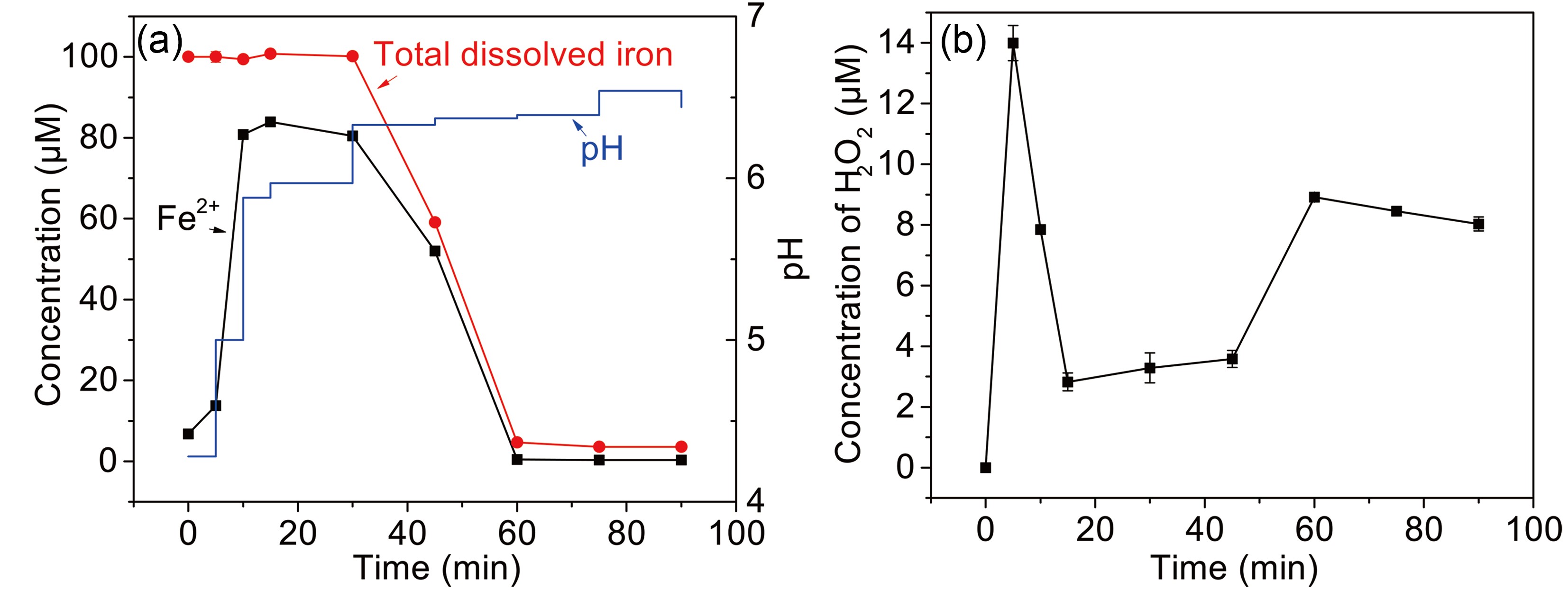
H2O2 and Fe(II) are the major long-lived oxidized active species in the photolysis of Fe(III)-Ox (Faust et al., 1993), and this determines the production of free radicals. As shown in Fig. 6a, Fe(II) concentration rapidly increases to 80% in 10 min, and then reduces to approximately 2% from 30 min to 60 min. Finally, Fe(II) concentration is maintained at an undetectable value in the remaining time. The total dissolved iron remains stable for 30 min and then quickly drops to only 5 μM left in the next 30 min. Fe(II) is usually formed by the photoreduction of
$ {\left[\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{3}\right]}^{3-} $ or$ {\left[\mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}\right]}^{-} $ and is finally consumed with H2O2 to form Fe(III). The accumulation of Fe(II) is due to the fast photoreduction rate. The change of total dissolved iron happens because the iron precipitates as insoluble Fe(OH)n(s), which is caused by decreasing oxalate concentration (Fig. A3) and increasing pH (Figs. 4. and A5). The change of pH is caused by the generation of$ {\mathrm{O}\mathrm{H}}^{-} $ in Fenton reaction. Fenton reaction can increase pH, as confirmed in a previous study (de Lima Perini et al., 2013). The pH increase can be attributed to the consumption of oxalate, leading to the release of Fe(III). The concentration of free oxalate changes, which might establish the acid-base reaction (de Luca et al., 2014). Otherwise, some organic acids are formed by the reaction of MACR and ·OH (Liu et al., 2009), which can decrease the solution pH.
Figure 6. (a) The change of Fe(II), total dissolved iron, and pH (b) H2O2 formation during the photooxidation of MACR in the Fe(III)-Ox system ([Fe(III)]0 = 100 µM, [Oxalate]0 = 1000 µM, [MACR]0 = 500 µM, pH 4.0 ± 0.1, air-saturated solution).
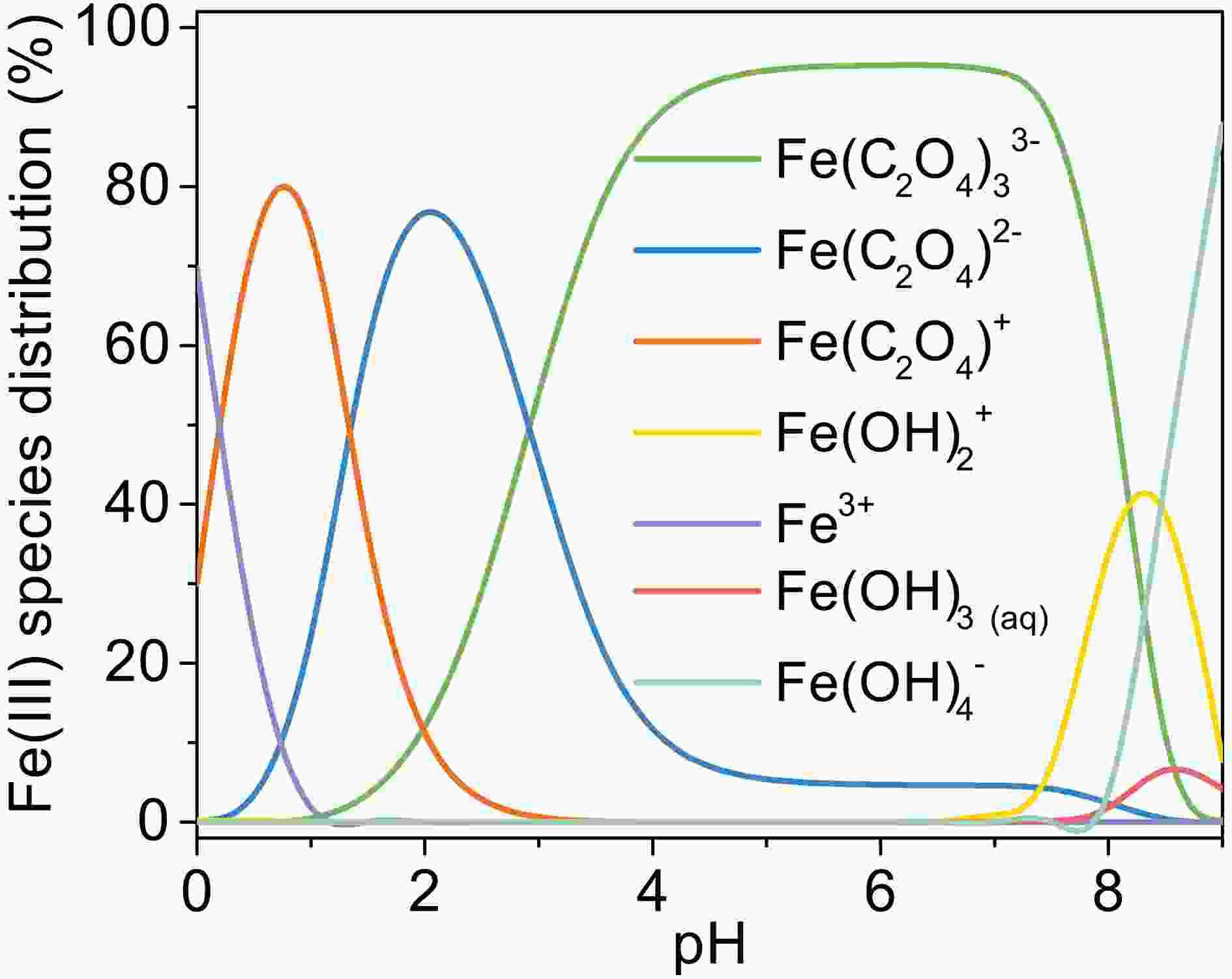
Figure A5. Change of Fe(III) species distribution with pH, [Fe(III)]0 = 100 µM, [Oxalate]0 = 1000 µM, 25°C.
The concentration of H2O2 increase in the first 5 min, then decreases in 5−45 min, and returns to increase after 45 minutes (Fig. 6b). H2O2 is the production of reaction of Fe(II) and
$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ /$ {{\mathrm{H}\mathrm{O}}_{2}^{\,\cdot\,}} $ , and$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ /$ {{\mathrm{H}\mathrm{O}}_{2}^{\,\cdot\,}} $ is from the photolysis of oxalate (Eqs. 4−9). According to Eqs. 9 and 10, low concentration Fe(II) is suitable for H2O2 accumulation, so H2O2 concentration increase first and then decrease with the increase of Fe(II) concentration in first 30 min. After 30 min, the increase in pH and the consumption of oxalate leads to the formation of$ {\mathrm{F}\mathrm{e}\left(\mathrm{O}\mathrm{H}\right)}_{{\rm{n}}} $ , which reduces the available Fe(II) and inhibits the rate of generation and consumption of H2O2 (Eqs. 9 and 10) simultaneously. At this stage, non-Fenton reactions might also play a role in the generation and consumption of H2O2 (Eqs. 12 and 13).The mechanism of Fe(III)-Ox photolysis is shown in Eqs. 4−11.
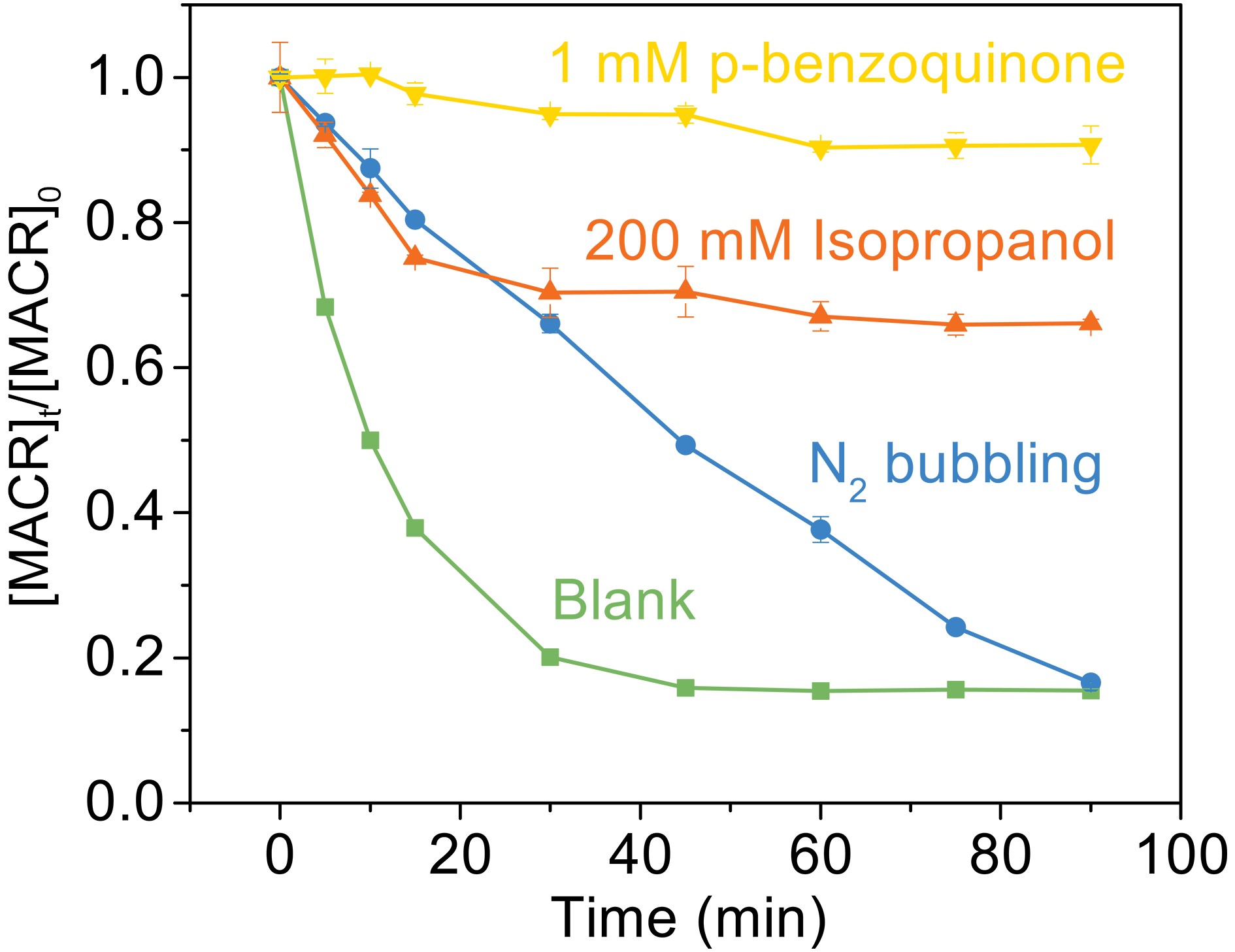
$ \mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{3}^{3-}} $ and$ \mathrm{F}\mathrm{e}\left(\mathrm{I}\mathrm{I}\mathrm{I}\right){{\left({\mathrm{C}}_{2}{\mathrm{O}}_{4}\right)}_{2}^{-}} $ are photolyzed to produce$ {{\mathrm{C}}_{2}\rm{O}_{\small 4}^{\overline {\,\cdot\,}} } $ (Eqs. 4−5), which forms$ {\mathrm{C}\rm{O}_{\small 2}^{\overline {\,\cdot\,}} } $ through decarboxylation (Eq. 6).$ {\mathrm{C}\rm{O}_{\small 2}^{\overline {\,\cdot\,}} } $ reacts with$ {\mathrm{O}}_{2} $ to produce$\rm{O}_{\small 2}^{\overline {\,\cdot\,}} ($ Eq. 7) and leads to the formation of$ {\mathrm{H}}_{2}{\mathrm{O}}_{2} $ (Eq. 9). ·OH can be produced continuously by the reaction between Fe(II) and H2O2 (Fenton reaction). In this study, 1 mM p-benzoquinone (BQ) was used as the scavenger of$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ (Patel and Willson, 1973), and 200 mM isopropanol (2-Pr) was used to quench ·OH. The experimental results are shown in Fig. 7. The addition of BQ and 2-Pr suppresses the oxidation of MACR, and only 10% and 30% oxidation remains after 90 min reaction, respectively. This indicates that$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ can also react with MACR in addition to ·OH. Nitrogen (N2) is introduced in the solution to eliminate oxygen (O2). However, it is difficult for the solution containing volatile MACR to remove all O2 if MACR is prevented from leaking simultaneously. So, the system still contains a part of O2. However, the N2 bubbling experiment proves that the conversion of O2 to$\rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ is an important speed-limiting step. A low concentration of O2 extends the time for the complete oxidation of MACR. The reaction of MACR with${{\mathrm{C}\rm{O}_{\small 2}^{\overline {\,\cdot\,}} }}$ is not considered in aerated water because of the rapid quenching reaction of${{\mathrm{C}\rm{O}_{\small 2}^{\overline {\,\cdot\,}} }}$ by O2 (Surdhar et al., 1989).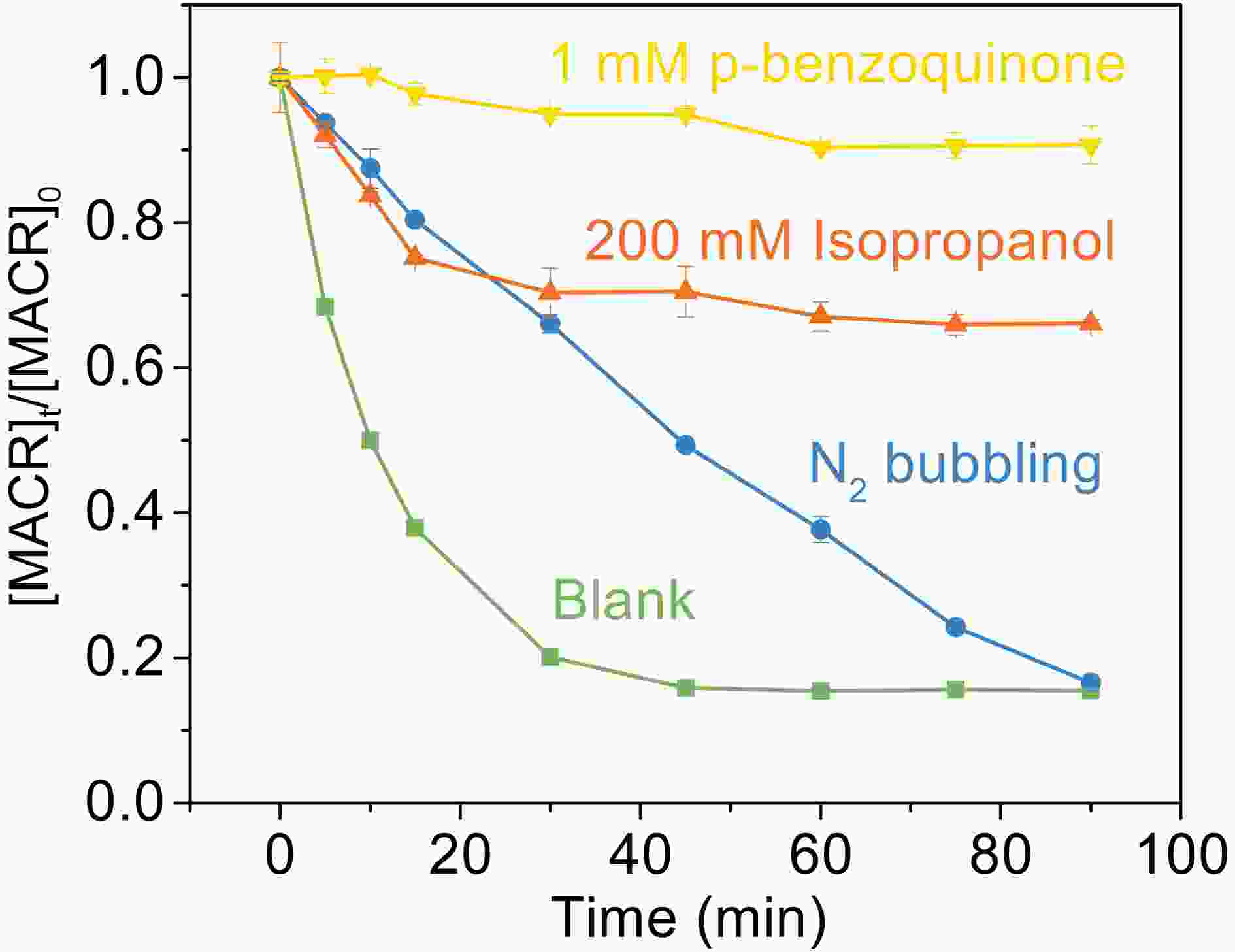
Figure 7. Effect of scavengers on the photooxidation of MACR in the Fe(III)-Ox system ([Fe(III)]0 = 100 µM, [Oxalate]0 = 1000 µM, [MACR]0 = 500 µM, pH 4.0 ± 0.1, air-saturated solution).
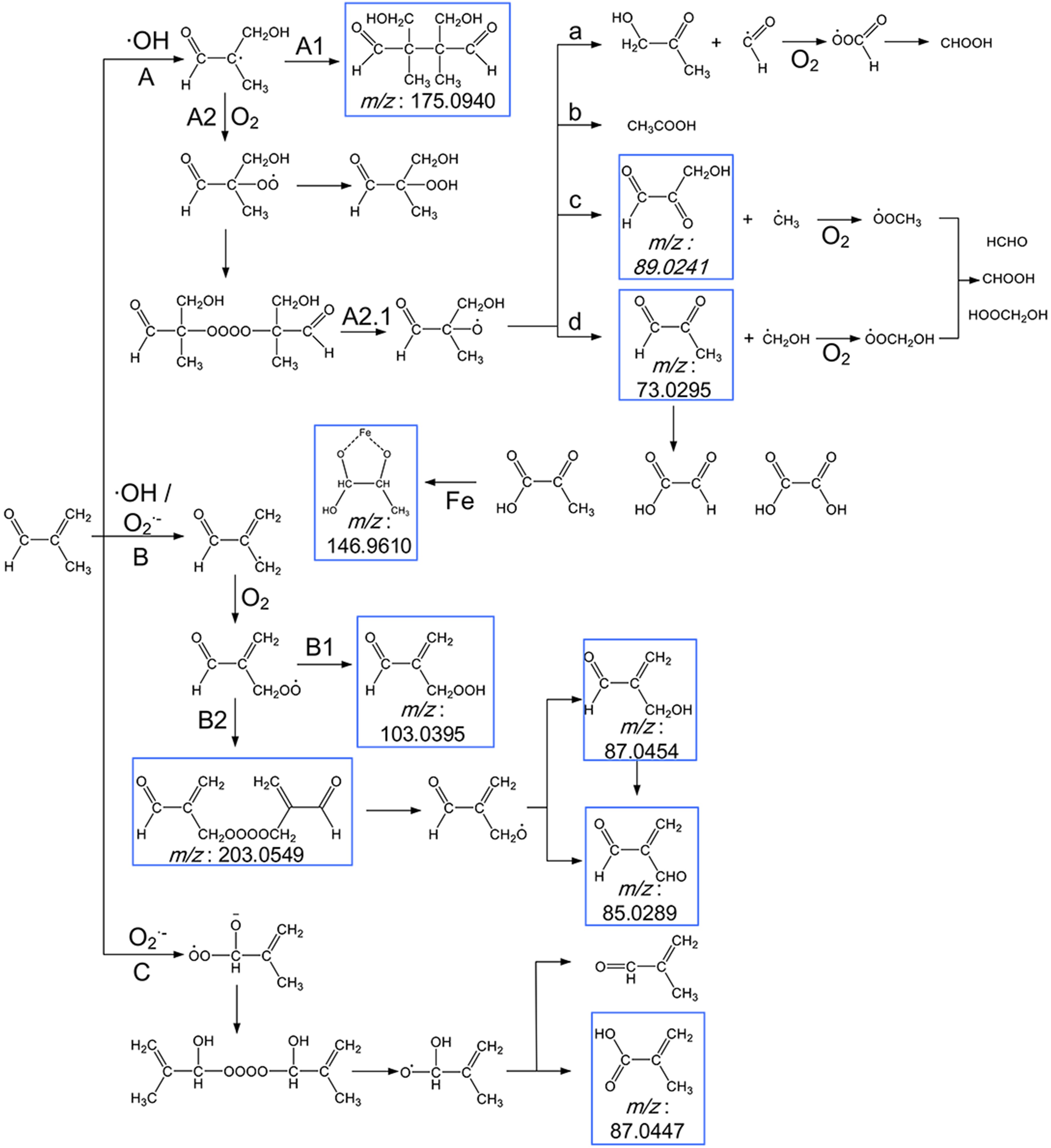
The proposed reaction pathways based on the observed products are shown in Fig. 8. Here, we present and discuss the three possible pathways oxidation of MACR in Fe(III)-Ox Fenton-like reaction can proceed: via addition on the C = C bond by ·OH (pathway A) (Liu et al., 2009; Zhang et al., 2010), H-abstraction of the methyl group by ·OH and
$\rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ (pathway B), nucleophilic addition on carbonyl group by$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ (pathway C) (Hayyan et al., 2016).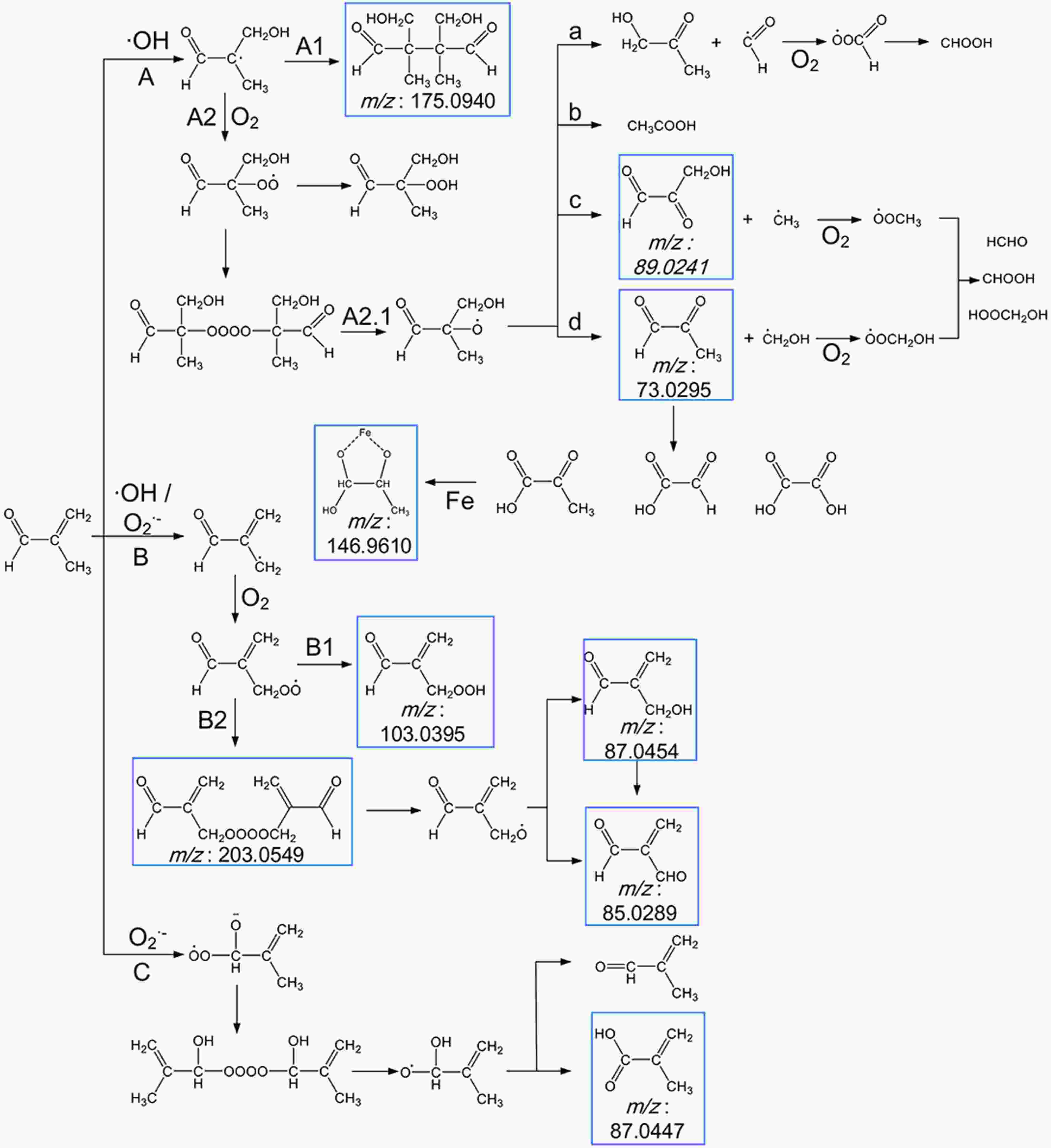
Figure 8. Scheme for the proposed reaction pathways of the photooxidation of MACR in the Fe(III)-Ox system. The molecular formulas in the blue frames are observed products.
The external addition of ·OH leads to the formation of an alkyl radical, which may form aldehyde or react with itself to form polymer C8H14O4 (Mass-to-charge ratio m/z = 175.0940) directly (pathway A1) or rapidly add to O2 to form peroxy radical (pathway A2). The peroxy radical reacts with itself to form an unstable tetroxide, then degrades to form alkoxy radical (pathway A2.1). This alkoxy radical undergoes decomposition and forms C3H4O2 (m/z = 73.0295), C3H4O3 (m/z = 89.0241), C3H6O2, and acetic acid (pathway A2.1a, b, c, d). The radical fragments generated during the decomposition can also react with O2 to form HCHO, CHOOH, and HOOCH2OH. The C3H4O2 (m/z = 73.0296) can also result in the formation of pyruvate, glyoxylate, and oxalate. ·OH and
$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ can attack MACR by hydrogen abstraction on the methyl group, leading to the formation of the alkyl radical (pathway B). Then by rapidly adding to O2 to form peroxy radical, which may form the corresponding carboxylic acid C4H6O3 (m/z = 103.0395) or react with itself to form a tetroxide C8H10O6 (m/z = 203.0549). Then C8H10O6 degrades to form alkoxy radical and results in the formation of C4H6O2 (m/z = 87.0454) and C4H4O2 (m/z = 85.0289).$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ may have a nucleophilic addition on carbonyl group (pathway C) and react with itself to form another tetroxide, and the corresponding aldehyde or carboxylic acid C4H6O2 (m/z = 87.0447) is generated through a similar approach as above.The formation of higher molecular weight compounds is observed, and the pathway of ·OH and
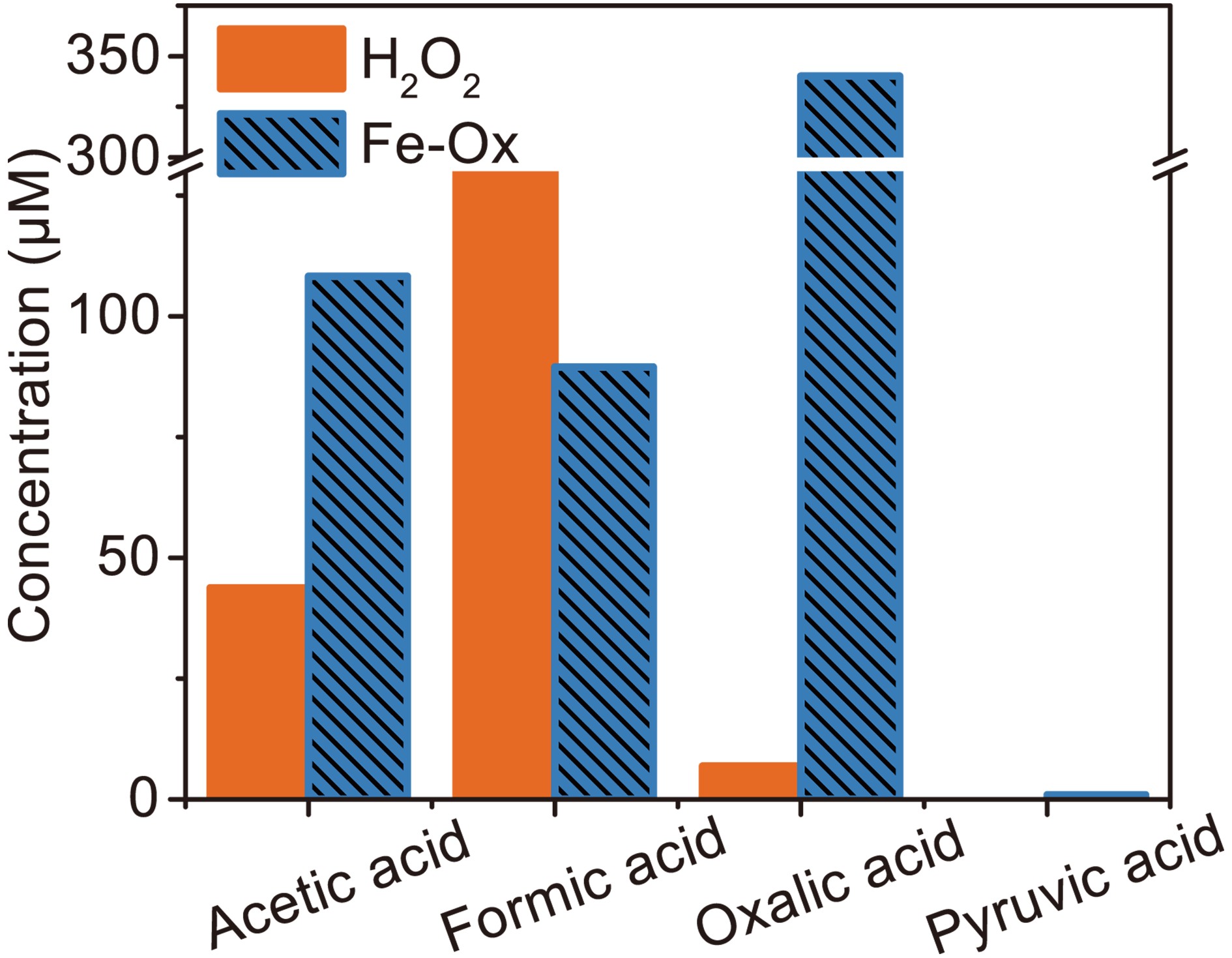
$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ to oxidize MACR is different. The photooxidation of MACR in the Fe(III)-Ox system is an important source of SOA in the atmosphere.Small organic acids are known to be the main oxidation products of MACR (El Haddad et al., 2009). So, IC was used to analyze the 60 min photooxidation sample of MACR in Fe(III)-Ox and H2O2 systems. Photolysis of H2O2 only produces ·OH, and the product is compared with the oxidation product from the Fe(III)-Ox system. Similar MACR photooxidation rates are obtained in 200 mM H2O2 after 60 min compared with the Fe(III)-Ox system. The results of IC measurements are shown in Fig. 9. Except for the oxalate added at the beginning, acetic, formic and pyruvic acids are detected in photolysis of MACR in the Fe(III)-Ox system, although pyruvic acid is not detected in the H2O2 system. The concentrations of small organic acids in the two systems have obvious differences. More acetic acid and less formic acid are obtained from the Fe(III)-Ox system. This may be because the oxidation mechanism of MACR in the two systems differs, and the involvement of
$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ influences the concentration of the products dramatically.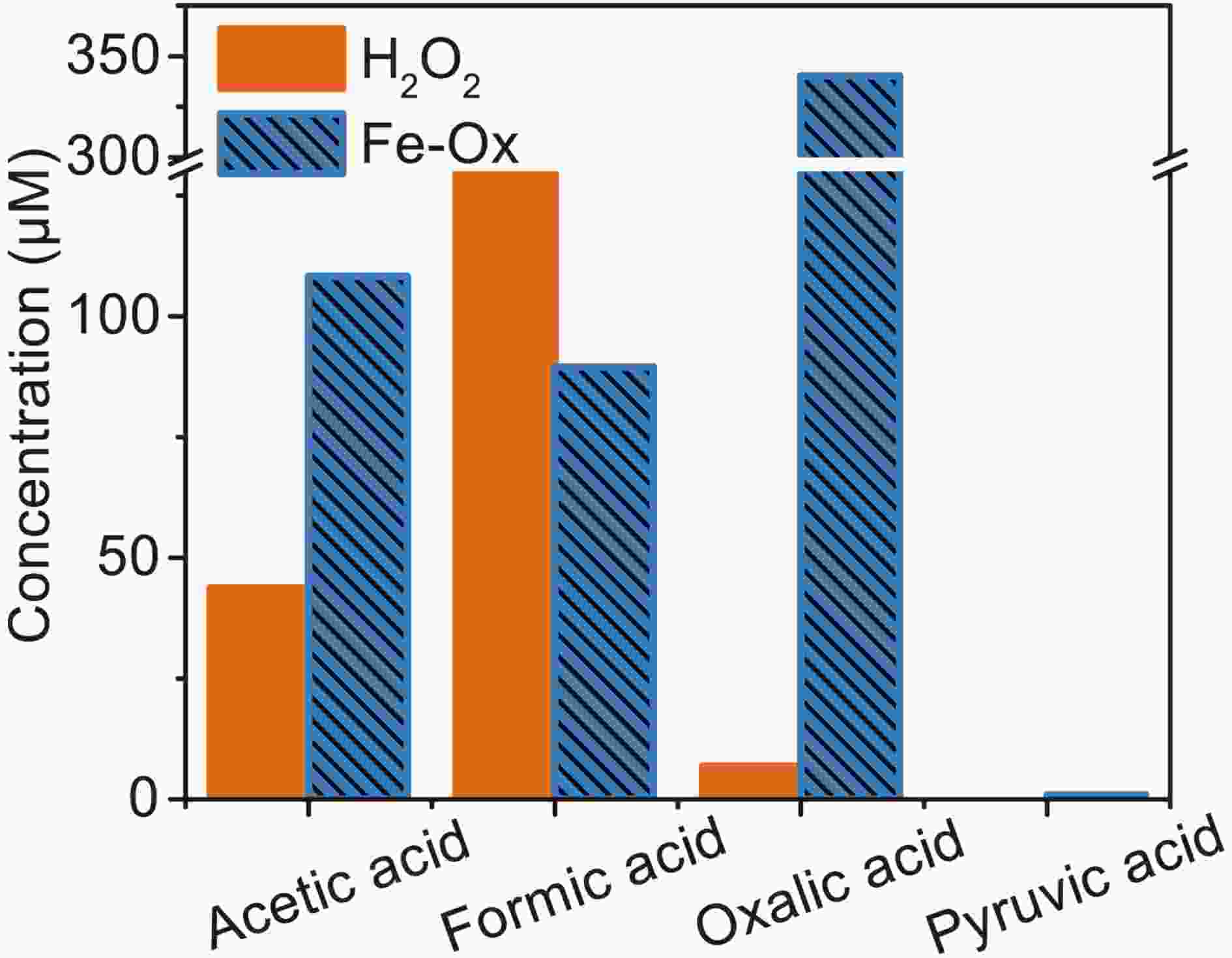
Figure 9. Concentration of small organic acids in the photooxidation of MACR in H2O2 and Fe(III)-Ox system at 60 min obtained by IC ([Fe(III)]0 = 100 µM, [Oxalate]0 = 1000 µM, [MACR]0 = 500 µM, pH 4.0 ± 0.1).
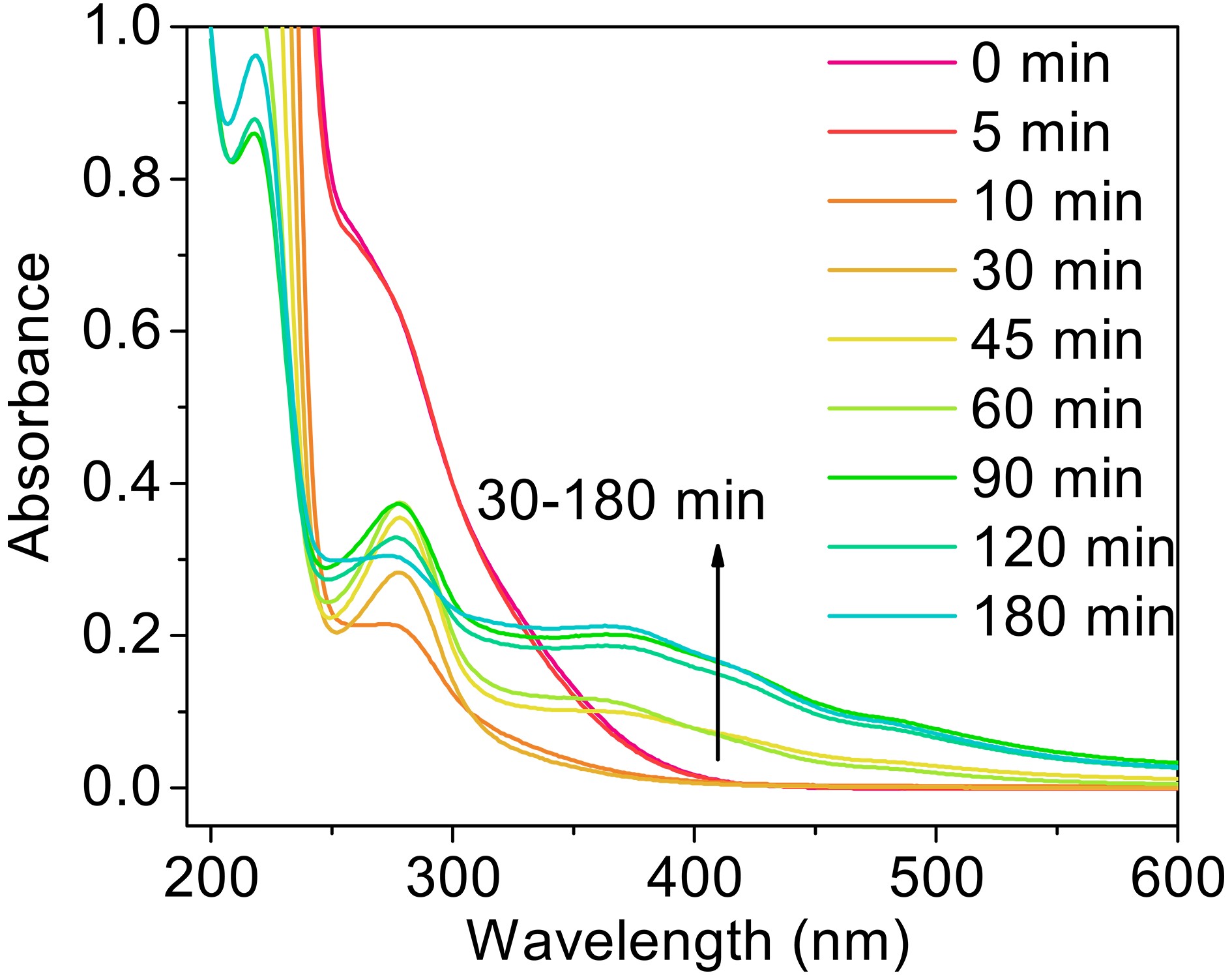
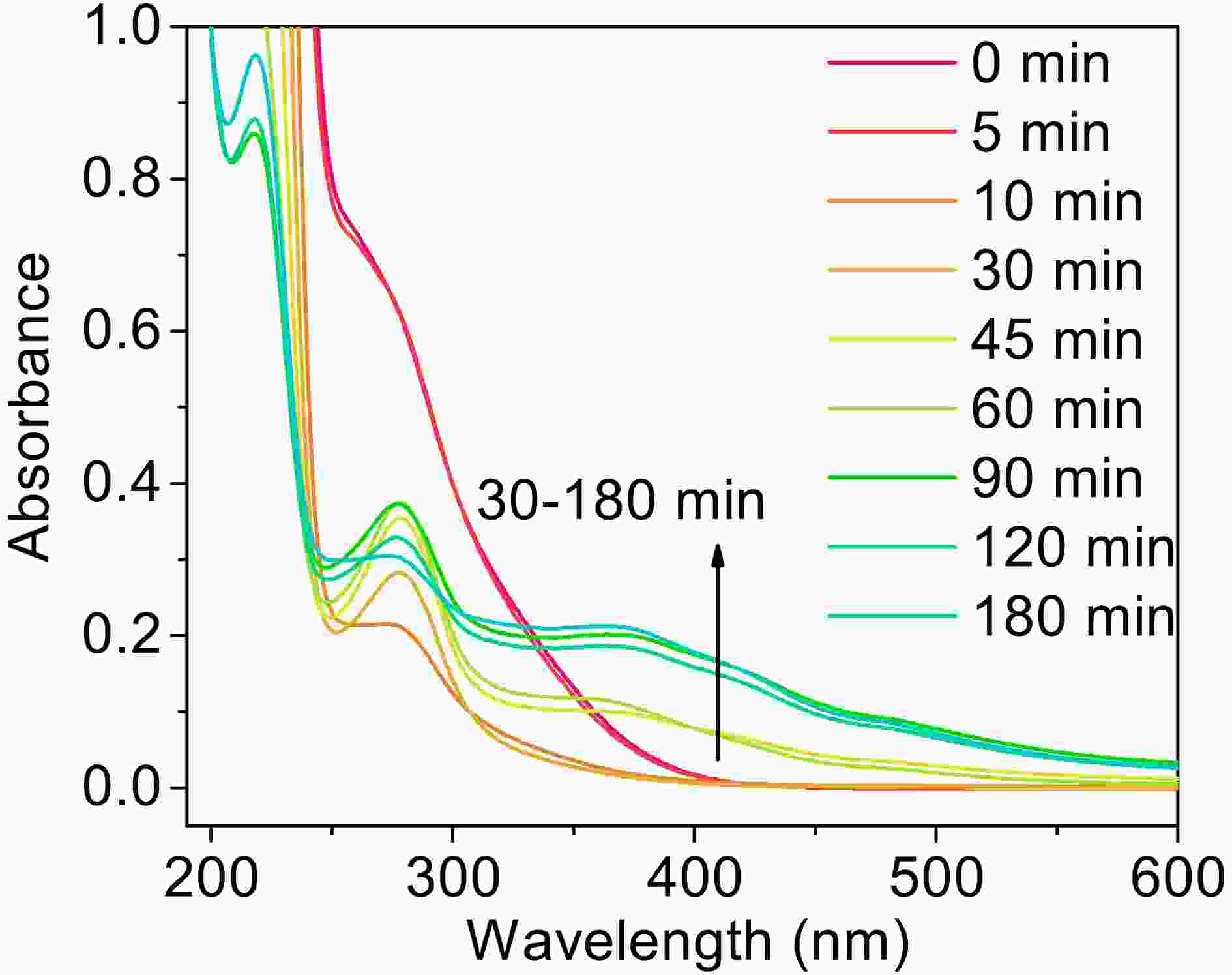
As shown in Fig. 10, the absorbance of the solution changes significantly with irradiation time. Two big absorbance peaks are found at around 280 nm and 310 nm at the beginning of the reaction, which from oxalate and MACR, respectively. The absorbance at 310 nm decreases in the first 30 min, and the absorbance of 280 nm relative to the nearby baseline decreases in the first 10 min, increases from 10 min to 60 min, then decreases from 60 min to 180 min. The absorbance of the region with a wavelength higher than 320 nm increases from 30 min to 150 min. The increasing absorbance at 280 nm might be due to the
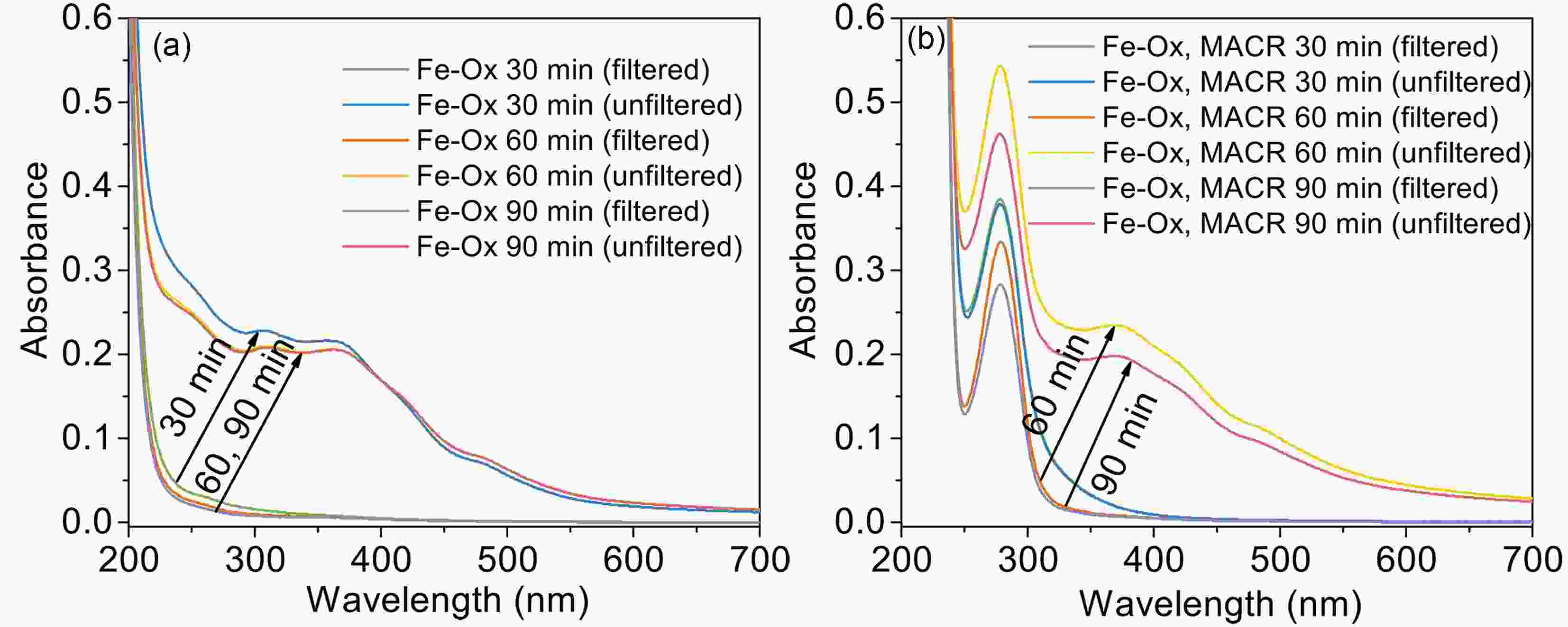
$ n\to {\mathrm{\pi }}^{\mathrm{*}} $ transition of the intermediate products, such as aldehyde or ketone. The increasing absorbance of other parts comes from the formation of yellow precipitates Fe(OH)n, which absorb light in the short wavelength, not from the oxidation products of MACR (Fig. A6). The clear yellow precipitates are produced in the late stage of the reaction. This finding indicates that the absorbance change caused by Fenton reaction might be an ignored factor, which would change the optical properties of aerosols and has implications for radiative forcing (Pillar-Little and Guzman, 2018).
3.1. The control experiments
3.2. Effect of oxalate concentration
3.3. Effect of Fe(III) concentration
3.4. Effects of solution pH
3.5. Effects of MACR concentration
3.6. Photooxidation mechanism of MACR in Fe(III)-Ox system
-
The photooxidation mechanism of MACR with Fe(III)-Ox in atmospheric water was studied through bulk experiments. MACR was barely directly photolyzed under simulated sunlight (>290 nm) irradiation, and it had little oxidation when Fe(III) was involved. A large amount of MACR was oxidized by ·OH and
$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ when Fe(III) and oxalate existed together under simulated sunlight irradiation conditions. This finding indicated that the presence of Fe(III) and oxalate greatly enhanced the oxidation ability in atmospheric water through the consumption of oxalate in Fe(III)-Ox. The recycling of Fe(II) and Fe(III) led to the generation of H2O2 and ·OH, which contributed to the oxidation capacity. The increase of pH during the reaction caused the production of$ \mathrm{F}\mathrm{e}{\left(\mathrm{O}\mathrm{H}\right)}_{{\rm{n}}} $ precipitate, which hindered the circulation of Fe(III) and Fe(II); it also caused the increase of the solution absorbance, which further affected the absorbance of aerosols and radiative forcing. The reaction pathways of the photooxidation of MACR in the Fe(III)-Ox system involving ·OH and$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ were proposed. The photooxidation of MACR in the Fe(III)-Ox system could produce a different concentration of small organic acids compared to the photolysis H2O2 system, which only contained ·OH. This further shows that$ \rm{O}_{\small 2}^{\overline {\,\cdot\,}} $ plays an important role in the oxidation of MACR. Additionally, oligomers with higher molecular weight were also observed in LC-QTOF-MS, and this finding indicates that MACR oxidation in the Fe(III)-Ox system could lead to SOA formation.Acknowledgements. The authors gratefully acknowledge financial support from the Ministry of Science and Technology of the People’s Republic of China (Grant Nos. 2017YFC0210005 and 2016YFE0112200).
APPENDIX





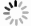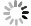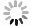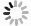
 DownLoad:
DownLoad:














































































 AAS Website
AAS Website 
 AAS WeChat
AAS WeChat